Abstract
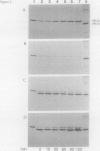
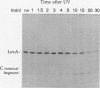
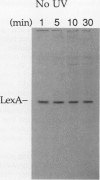
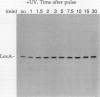
The inducible SOS response for DNA repair and mutagenesis in the bacterium Bacillus subtilis resembles the extensively characterized SOS system of Escherichia coli. In this report, we demonstrate that the cellular repressor of the E. coli SOS system, the LexA protein, is specifically cleaved in B. subtilis following exposure of the cells to DNA-damaging treatments that induce the SOS response. The in vivo cleavage of LexA is dependent upon the functions of the E. coli RecA protein homolog in B. subtilis (B. subtilis RecA) and results in the same two cleavage fragments as produced in E. coli cells following the induction of the SOS response. We also show that a mutant form of the E. coli RecA protein (RecA430) can partially substitute for the nonfunctional cellular RecA protein in the B. subtilis recA4 mutant, in a manner consistent with its known activities and deficiencies in E. coli. RecA430 protein, which has impaired repressor cleaving (LexA, UmuD, and bacteriophage lambda cI) functions in E.coli, partially restores genetic exchange to B. subtilis recA4 strains but, unlike wild-type E. coli RecA protein, is not capable of inducing SOS functions (expression of DNA damage-inducible [din::Tn917-lacZ] operons or RecA synthesis) in B. subtilis in response to DNA-damaging agents or those functions that normally accompany the development of physiological competence. Our results provide support for the existence of a cellular repressor in B. subtilis that is functionally homologous to the E. coli LexA repressor and suggest that the mechanism by which B. subtilis RecA protein (like RecA of E. coli) becomes activated to promote the induction of the SOS response is also conserved.
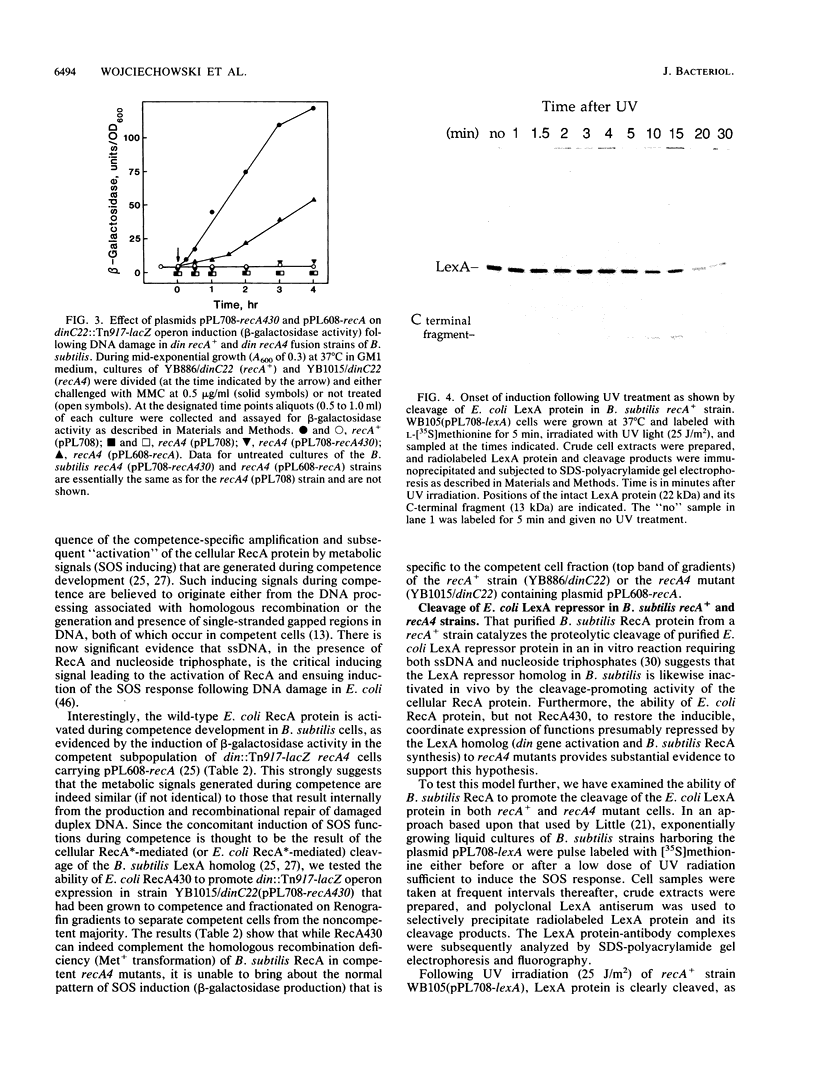
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Cheo D. L., Bayles K. W., Yasbin R. E. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J Bacteriol. 1991 Mar;173(5):1696–1703. doi: 10.1128/jb.173.5.1696-1703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elespuru R. K. Inducible responses to DNA damage in bacteria and mammalian cells. Environ Mol Mutagen. 1987;10(1):97–116. doi: 10.1002/em.2850100111. [DOI] [PubMed] [Google Scholar]
- Ennis D. G., Ossanna N., Mount D. W. Genetic separation of Escherichia coli recA functions for SOS mutagenesis and repressor cleavage. J Bacteriol. 1989 May;171(5):2533–2541. doi: 10.1128/jb.171.5.2533-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986 Mar;165(3):715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Structural features of DNA in competent Bacillus subtilis. Mol Gen Genet. 1971;113(4):316–330. doi: 10.1007/BF00272332. [DOI] [PubMed] [Google Scholar]
- Hill S. A., Little J. W. Allele replacement in Escherichia coli by use of a selectable marker for resistance to spectinomycin: replacement of the lexA gene. J Bacteriol. 1988 Dec;170(12):5913–5915. doi: 10.1128/jb.170.12.5913-5915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Nakatani T., Hase T., Matsubara H., Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981 Dec;27(3 Pt 2):515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
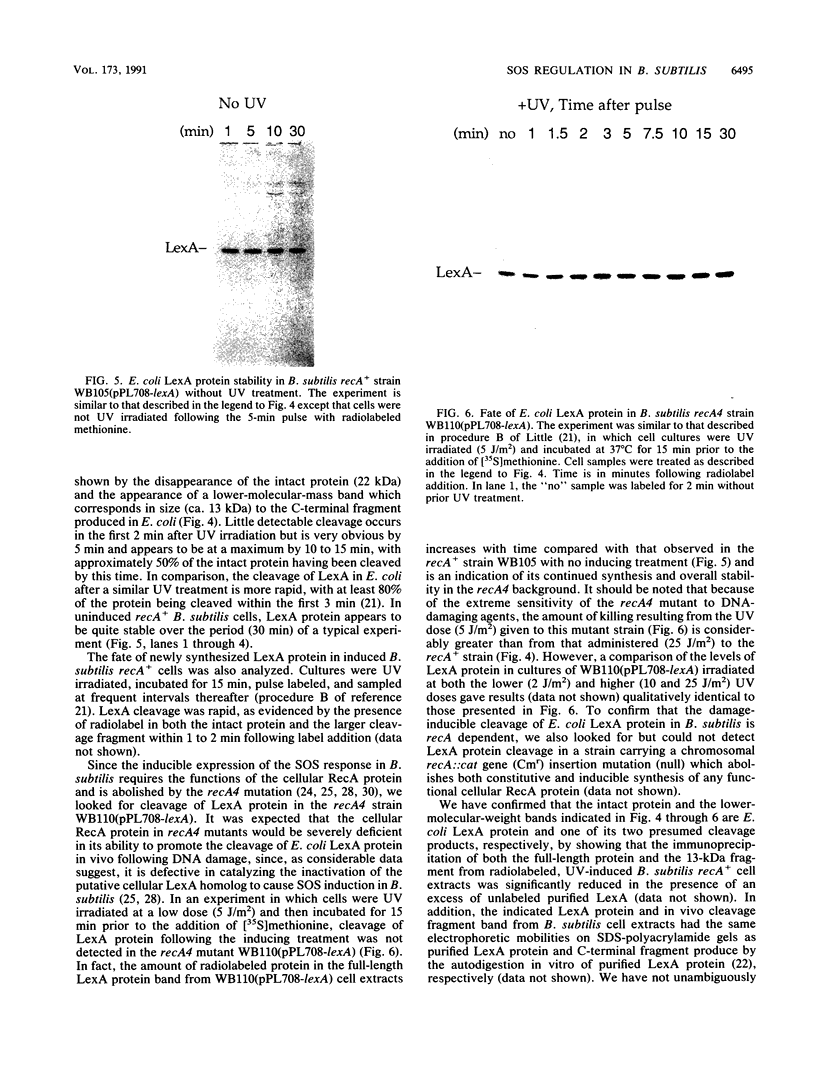
- Koomey J. M., Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987 Feb;169(2):790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. Isolation of recombinant plasmids and phage carrying the lexA gene of Escherichia coli K-12. Gene. 1980 Aug;10(3):237–247. doi: 10.1016/0378-1119(80)90053-0. [DOI] [PubMed] [Google Scholar]
- Little J. W. The SOS regulatory system: control of its state by the level of RecA protease. J Mol Biol. 1983 Jul 15;167(4):791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Induction of the Bacillus subtilis SOS-like response by Escherichia coli RecA protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5204–5208. doi: 10.1073/pnas.83.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Love P. E., Yasbin R. E. Competence-specific induction of the Bacillus subtilis RecA protein analog: evidence for dual regulation of a recombination protein. J Bacteriol. 1989 May;171(5):2318–2322. doi: 10.1128/jb.171.5.2318-2322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Love P. E., Yasbin R. E., Roberts J. W. SOS-like induction in Bacillus subtilis: induction of the RecA protein analog and a damage-inducible operon by DNA damage in Rec+ and DNA repair-deficient strains. J Bacteriol. 1988 Apr;170(4):1467–1474. doi: 10.1128/jb.170.4.1467-1474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- Lu C., Echols H. RecA protein and SOS. Correlation of mutagenesis phenotype with binding of mutant RecA proteins to duplex DNA and LexA cleavage. J Mol Biol. 1987 Aug 5;196(3):497–504. doi: 10.1016/0022-2836(87)90027-1. [DOI] [PubMed] [Google Scholar]
- Marrero R., Yasbin R. E. Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in B. subtilis. J Bacteriol. 1988 Jan;170(1):335–344. doi: 10.1128/jb.170.1.335-344.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mongkolsuk S., Chiang Y. W., Reynolds R. B., Lovett P. S. Restriction fragments that exert promoter activity during postexponential growth of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1399–1406. doi: 10.1128/jb.155.3.1399-1406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C., Bryant D. A., Porter R. D., de Marsac N. T. Molecular cloning and characterization of the recA gene from the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol. 1987 Jun;169(6):2739–2747. doi: 10.1128/jb.169.6.2739-2747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Ossanna N., Thliveris A. T., Ennis D. G., Mount D. W. Derepression of specific genes promotes DNA repair and mutagenesis in Escherichia coli. J Bacteriol. 1988 Jan;170(1):1–4. doi: 10.1128/jb.170.1.1-4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protić M., Roilides E., Levine A. S., Dixon K. Enhancement of DNA repair capacity of mammalian cells by carcinogen treatment. Somat Cell Mol Genet. 1988 Jul;14(4):351–357. doi: 10.1007/BF01534643. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Two mutations that alter the regulatory activity of E. coli recA protein. Nature. 1981 Apr 2;290(5805):422–424. doi: 10.1038/290422a0. [DOI] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W. Specific Saccharomyces cerevisiae genes are expressed in response to DNA-damaging agents. Mol Cell Biol. 1985 Jan;5(1):75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassanfar M., Roberts J. W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990 Mar 5;212(1):79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- Schmitt J. J., Cohen B. N. Quantitative isolation of DNA restriction fragments from low-melting agarose by Elutip-d affinity chromatography. Anal Biochem. 1983 Sep;133(2):462–464. doi: 10.1016/0003-2697(83)90109-4. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Goodwin P. A. Interspecies regulation of the SOS response by the E. coli lexA+ gene. Mutat Res. 1985 May;145(3):103–106. doi: 10.1016/0167-8817(85)90015-x. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Spikes D., Griffin K. Characterization of the rec-1 gene of Haemophilus influenzae and behavior of the gene in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3876–3881. doi: 10.1128/jb.170.9.3876-3881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Isolation of protease-proficient, recombinase-deficient recA mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):688–695. doi: 10.1128/jb.163.2.688-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Countryman J. K., Howard-Flanders P. Purification and properties of the recA protein of Proteus mirabilis. Comparison with Escherichia coli recA protein; specificity of interaction with single strand binding protein. J Biol Chem. 1983 Apr 10;258(7):4648–4654. [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski M. F., Hoelzer M. A., Michod R. E. DNA repair and the evolution of transformation in Bacillus subtilis. II. Role of inducible repair. Genetics. 1989 Mar;121(3):411–422. doi: 10.1093/genetics/121.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M., de Vries S. C., Venema G. Cloning and expression of the Escherichia coli recA gene in Bacillus subtilis. Gene. 1983 Nov;25(2-3):301–308. doi: 10.1016/0378-1119(83)90234-2. [DOI] [PubMed] [Google Scholar]