Abstract
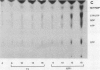
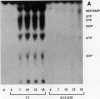
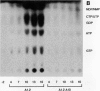
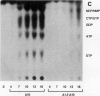
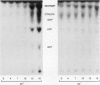
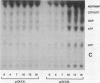
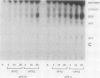
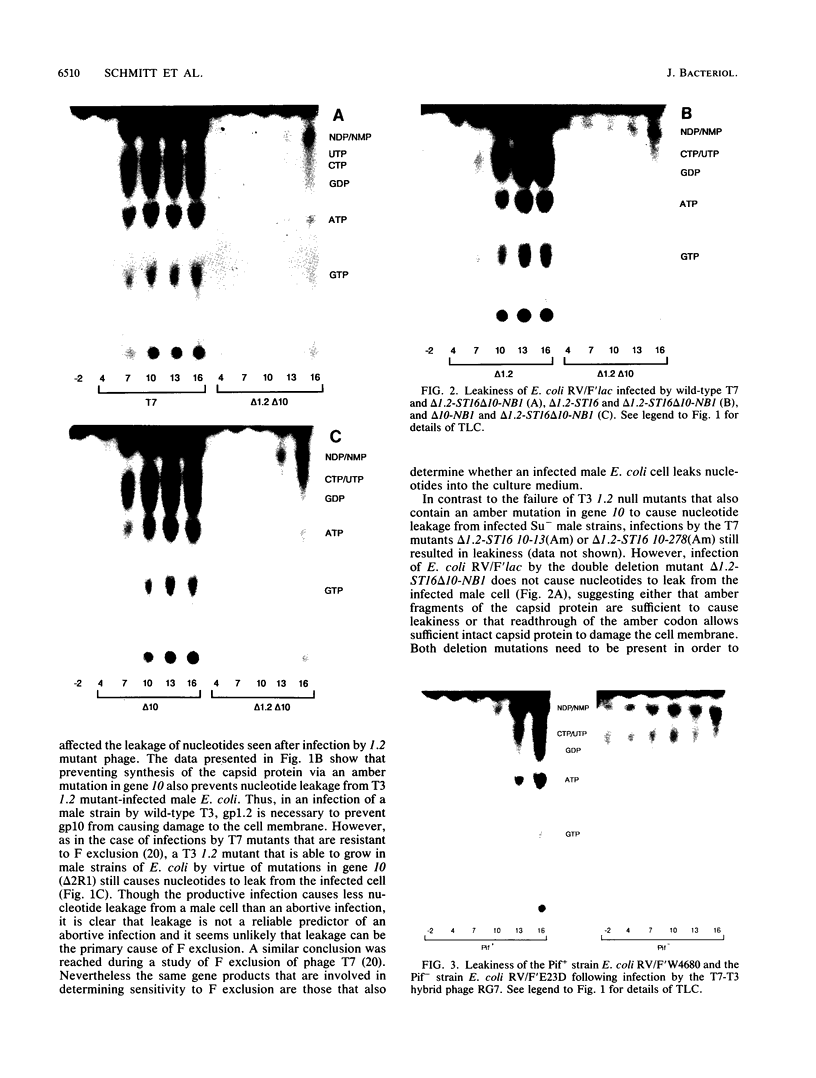
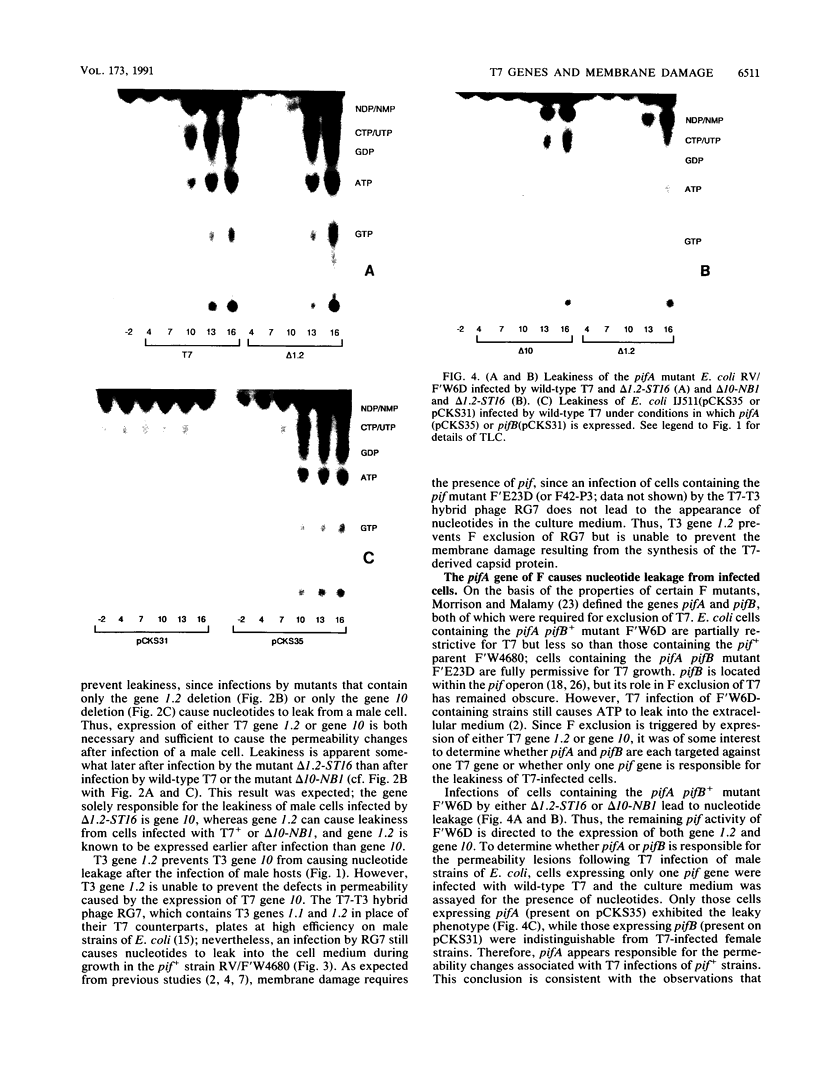
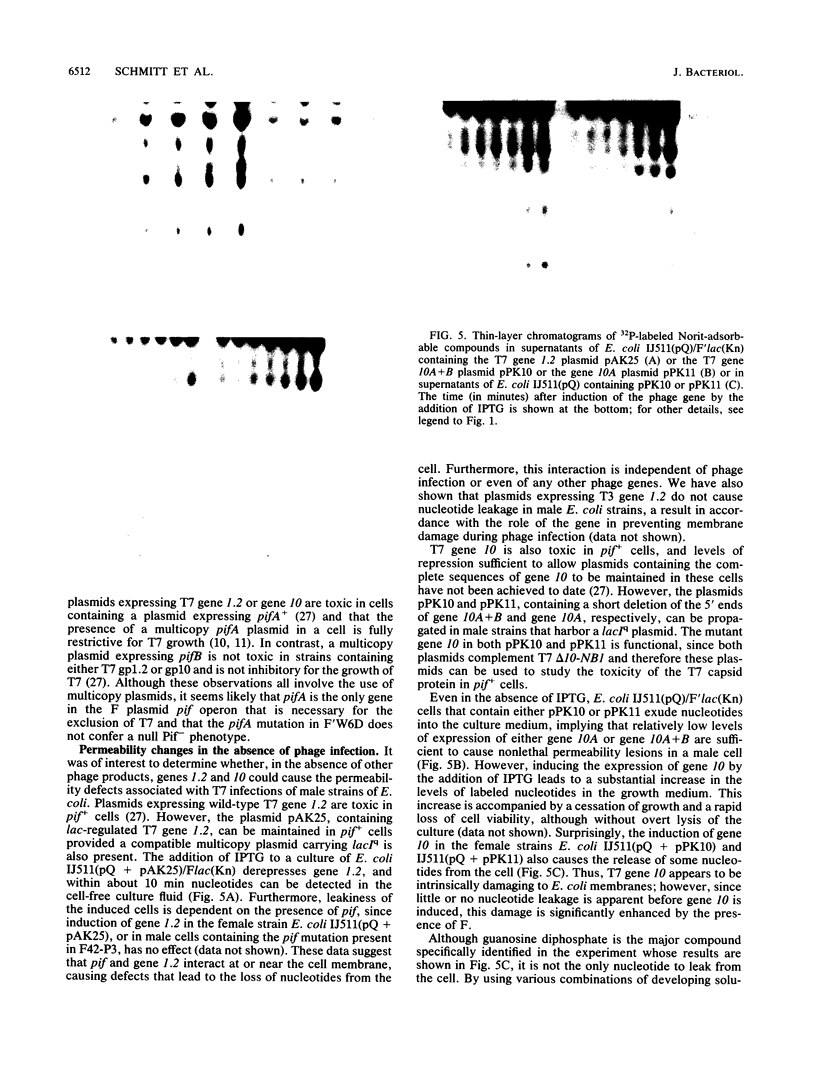
Infections of F plasmid-containing strains of Escherichia coli by bacteriophage T7 result in membrane damage that allows nucleotides to exude from the infected cell into the culture medium. Only pifA of the F pif operon is necessary for "leakiness" of the T7-infected cell. Expression of either T7 gene 1.2 or gene 10 is sufficient to cause leakiness, since infections by phage containing null mutations in both of these genes do not result in permeability changes of the F-containing cell. Even in the absence of phage infection, expression from plasmids of either gene 1.2 or 10 can cause permeability changes, particularly of F plasmid-containing cells. In contrast, gene 1.2 of the related bacteriophage T3 prevents leakiness of the infected cell. In the absence of T3 gene 1.2 function, expression of gene 10 causes membrane damage that allows nucleotides to leak from the cell. Genes 1.2 and 10 of both T3 and T7 are the two genes involved in determining resistance or sensitivity to F exclusion; F exclusion and leakiness of the phage-infected cell are therefore closely related phenomena. However, since leakiness of the infected cell does not necessarily result in phage exclusion, it cannot be used as a predictor of an abortive infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. J., Molineux I. J. Defective transcription of the right end of bacteriophage T7 DNA during an abortive infection of F plasmid-containing Escherichia coli. J Bacteriol. 1991 Feb;173(3):947–954. doi: 10.1128/jb.173.3.947-954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Gorini L. Growth of bacteriophages MS2 and T7 on streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1975 Feb;121(2):670–674. doi: 10.1128/jb.121.2.670-674.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Condreay J. P., Molineux I. J. Synthesis of the capsid protein inhibits development of bacteriophage T3 mutants that abortively infect F plasmid-containing cells. J Mol Biol. 1989 Jun 5;207(3):543–554. doi: 10.1016/0022-2836(89)90463-4. [DOI] [PubMed] [Google Scholar]
- Condreay J. P., Wright S. E., Molineux I. J. Nucleotide sequence and complementation studies of the gene 10 region of bacteriophage T3. J Mol Biol. 1989 Jun 5;207(3):555–561. doi: 10.1016/0022-2836(89)90464-6. [DOI] [PubMed] [Google Scholar]
- Cram D., Ray A., Skurray R. Molecular analysis of F plasmid pif region specifying abortive infection of T7 phage. Mol Gen Genet. 1984;197(1):137–142. doi: 10.1007/BF00327934. [DOI] [PubMed] [Google Scholar]
- Cram H. K., Cram D., Skurray R. F plasmid pif region: Tn1725 mutagenesis and polypeptide analysis. Gene. 1984 Dec;32(1-2):251–254. doi: 10.1016/0378-1119(84)90053-2. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Huber H. E., Beauchamp B. B., Richardson C. C. Escherichia coli dGTP triphosphohydrolase is inhibited by gene 1.2 protein of bacteriophage T7. J Biol Chem. 1988 Sep 25;263(27):13549–13556. [PubMed] [Google Scholar]
- Miller J. F., Lanka E., Malamy M. H. F factor inhibition of conjugal transfer of broad-host-range plasmid RP4: requirement for the protein product of pif operon regulatory gene pifC. J Bacteriol. 1985 Sep;163(3):1067–1073. doi: 10.1128/jb.163.3.1067-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Malamy M. H. Identification of the pifC gene and its role in negative control of F factor pif gene expression. J Bacteriol. 1983 Oct;156(1):338–347. doi: 10.1128/jb.156.1.338-347.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux I. J. Host-parasite interactions: recent developments in the genetics of abortive phage infections. New Biol. 1991 Mar;3(3):230–236. [PubMed] [Google Scholar]
- Molineux I. J., Schmitt C. K., Condreay J. P. Mutants of bacteriophage T7 that escape F restriction. J Mol Biol. 1989 Jun 5;207(3):563–574. doi: 10.1016/0022-2836(89)90465-8. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Spence J. L. Virus-plasmid interactions: mutants of bacteriophage T3 that abortively infect plasmid F-containing (F+) strains of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1465–1469. doi: 10.1073/pnas.81.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Blumberg D. D., Malamy M. H. T7 protein synthesis in F' episome-containing cells: assignment of specific proteins to three translational groups. J Virol. 1974 Feb;13(2):386–393. doi: 10.1128/jvi.13.2.386-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Myers J. A., Beauchamp B. B., White J. H., Richardson C. C. Purification and characterization of the gene 1.2 protein of bacteriophage T7. J Biol Chem. 1987 Apr 15;262(11):5280–5287. [PubMed] [Google Scholar]
- Remes B., Elseviers D. Adenosine 5'-triphosphate leakage does not cause abortive infection of bacteriophage T7 in male Escherichia coli. J Bacteriol. 1980 Aug;143(2):1054–1056. doi: 10.1128/jb.143.2.1054-1056.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G. S., Cooney R., Malamy M. H. Cloning of the pif region of the F sex factor and identification of a pif protein product. J Bacteriol. 1983 Jul;155(1):254–264. doi: 10.1128/jb.155.1.254-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C. K., Molineux I. J. Expression of gene 1.2 and gene 10 of bacteriophage T7 is lethal to F plasmid-containing Escherichia coli. J Bacteriol. 1991 Feb;173(4):1536–1543. doi: 10.1128/jb.173.4.1536-1543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Beck P. J., Kearney C. A., Spence J. L., DiGiovanni D., Condreay J. P., Molineux I. J. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J Mol Biol. 1987 Feb 5;193(3):479–495. doi: 10.1016/0022-2836(87)90261-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]