Abstract
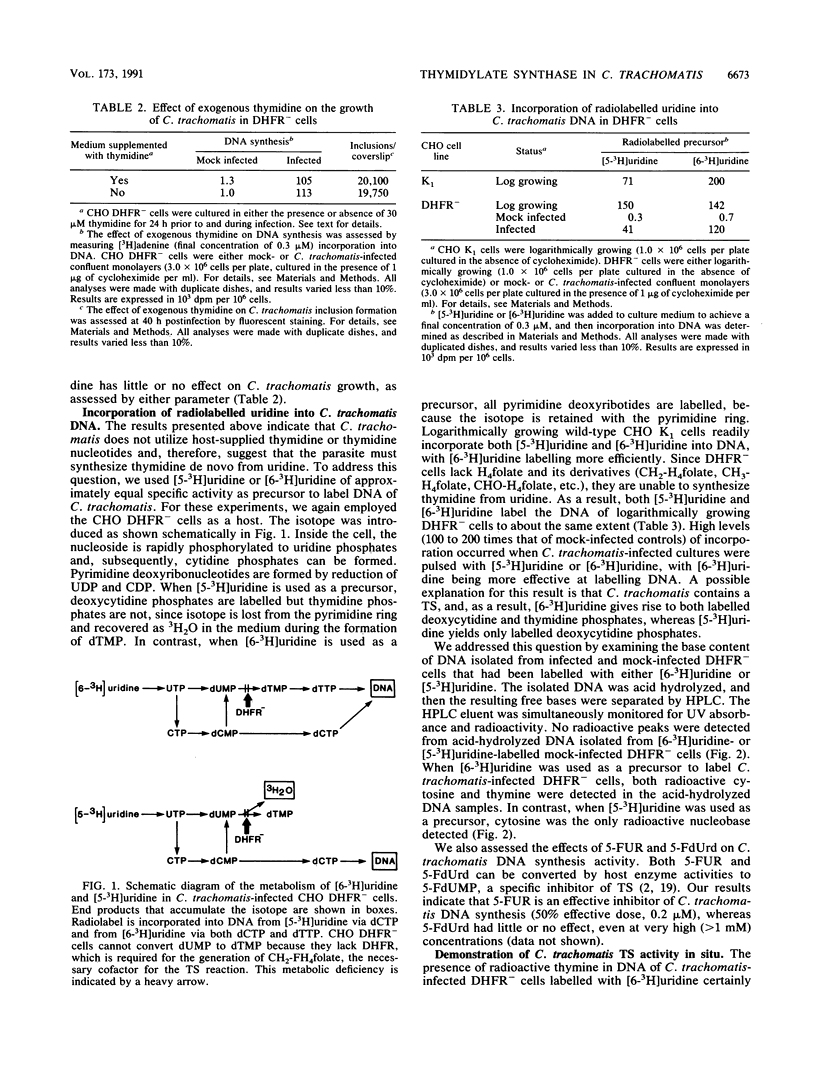
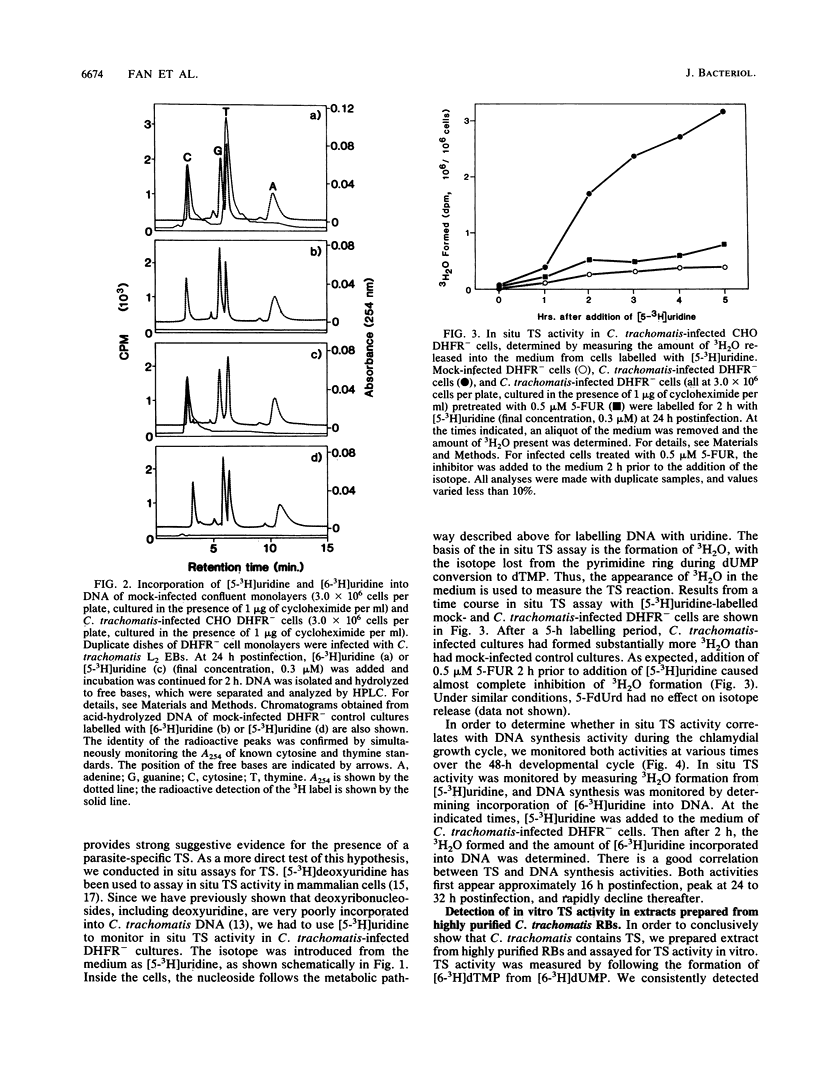
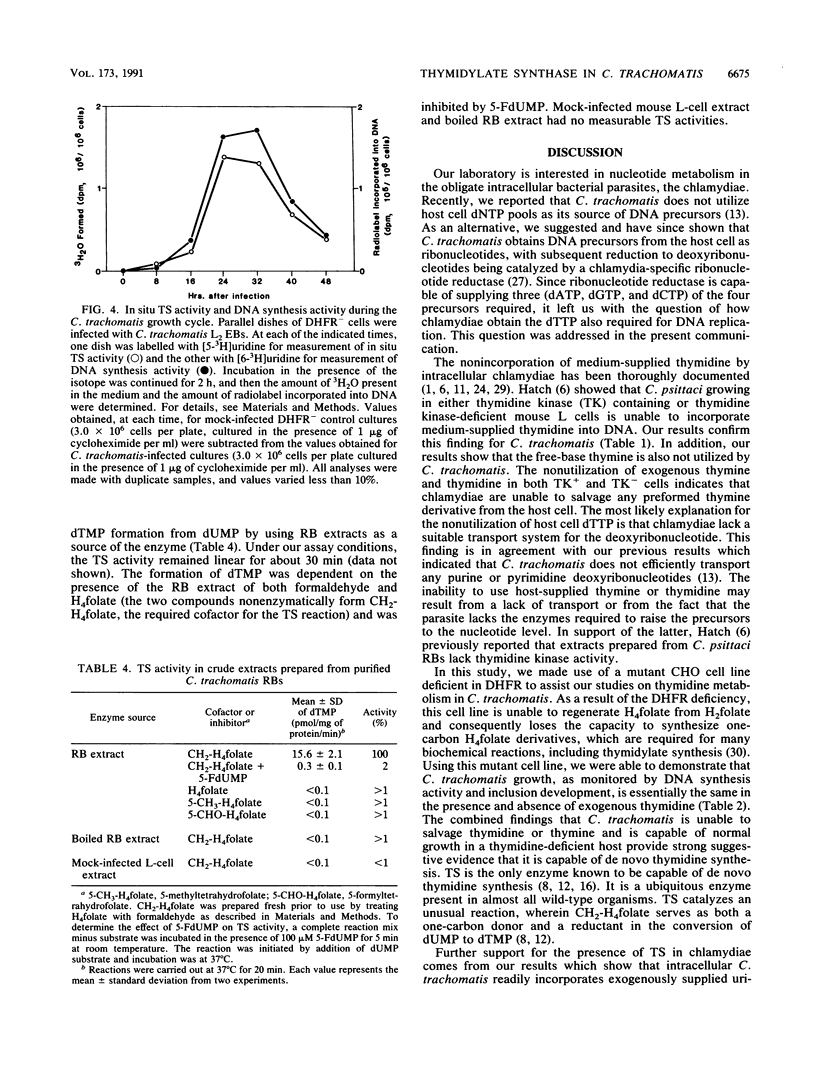
Since eucaryotic cell-derived thymidine or thymidine nucleotides are not incorporated into Chlamydia trachomatis DNA, we hypothesized that C. trachomatis must obtain dTTP for DNA synthesis by converting dUMP to dTMP. In most cells, this reaction is catalyzed by thymidylate synthase (TS) and requires 5,10-methylenetetrahydrofolate as a cofactor. We used C. trachomatis serovar L2 and a mutant CHO K1 cell line with a genetic deficiency in folate metabolism as a host for chlamydial growth. This cell line lacks a functional dihydrofolate reductase (DHFR) gene and, as a result, is unable to carry out de novo synthesis of dTTP. C. trachomatis inclusions form normally when DHFR- cells are starved for thymidine 24 h prior to and during the course of infection. When [6-3H]uridine is used as a precursor to label C. trachomatis-infected CHO DHFR- cells, radiolabel is readily incorporated into chlamydia-specific DNA. When DNA from [6-3H]uridine-labelled infected cultures is acid hydrolyzed and subjected to high-performance liquid chromatography analysis, radiolabel is detected in thymine and cytosine nucleobases. By using the DHFR- cell line as a host and [5-3H]uridine as a precursor, we could monitor intracellular C. trachomatis TS activity simply by following the formation of tritiated water. There is a good correlation between in situ TS activity and DNA synthesis activity during the chlamydial growth cycle. In addition, both C. trachomatis-specific DNA synthesis and 3H2O release are inhibited by exogenously added 5-fluorouridine but not by 5-fluorodeoxyuridine. Finally, we demonstrated in vitro TS activity in crude extracts prepared from highly purified C. trachomatis reticulate bodies. The activity is dependent on the presence of methylenetetrahydrofolic acid and can be inhibited with 5-fluoro-dUMP. Taken together, these results indicate that C. trachomatis contains a TS for the synthesis of dTMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. K., Liebhaber H. Deoxyribonucleic acid synthesis, cell cycle progression, and division of Chlamydia-infected HeLa 229 cells. Infect Immun. 1979 Jun;24(3):953–957. doi: 10.1128/iai.24.3.953-957.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Fritz H. J., Doerfler W. Quantitative determination of 5-methylcytosine in DNA by reverse-phase high-performance liquid chromatography. Anal Biochem. 1983 Nov;135(1):165–171. doi: 10.1016/0003-2697(83)90746-7. [DOI] [PubMed] [Google Scholar]
- Fraiz J., Jones R. B. Chlamydial infections. Annu Rev Med. 1988;39:357–370. doi: 10.1146/annurev.me.39.020188.002041. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of exogenous thymidine by Chlamydia psittaci growing in the thymidine kinase-containing and thymidine kinase-deficient L cells. J Bacteriol. 1976 Feb;125(2):706–712. doi: 10.1128/jb.125.2.706-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanetich K. M., Santi D. V. Biomedical science and the third world. Under the volcano. Recent studies on thymidylate synthase. Ann N Y Acad Sci. 1989;569:201–210. doi: 10.1111/j.1749-6632.1989.tb27370.x. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Krungkrai J., Yuthavong Y., Webster H. K. High-performance liquid chromatographic assay for thymidylate synthase from the human malaria parasite, Plasmodium falciparum. J Chromatogr. 1989 Jan 27;487(1):51–59. doi: 10.1016/s0378-4347(00)83006-6. [DOI] [PubMed] [Google Scholar]
- Lin H. S. Inhibition of thymidine kinase activity and deoxyribonucleic acid synthesis in L cells infected with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2054–2065. doi: 10.1128/jb.96.6.2054-2065.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., Maley G. F. A tale of two enzymes, deoxycytidylate deaminase and thymidylate synthase. Prog Nucleic Acid Res Mol Biol. 1990;39:49–80. doi: 10.1016/s0079-6603(08)60623-6. [DOI] [PubMed] [Google Scholar]
- McClarty G., Tipples G. In situ studies on incorporation of nucleic acid precursors into Chlamydia trachomatis DNA. J Bacteriol. 1991 Aug;173(16):4922–4931. doi: 10.1128/jb.173.16.4922-4931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radparvar S., Houghton P. J., Germain G., Pennington J., Rahman A., Houghton J. A. Cellular pharmacology of 5-fluorouracil in a human colon adenocarcinoma cell line selected for thymidine kinase deficiency. Biochem Pharmacol. 1990 Jun 1;39(11):1759–1765. doi: 10.1016/0006-2952(90)90122-2. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Moroson B. A., Bertino J. R. Regulation of thymidylate synthetase in mouse leukemia cells (L1210). J Biol Chem. 1980 Feb 25;255(4):1305–1311. [PubMed] [Google Scholar]
- STARR T. J., SHARON N. AUTORADIOGRAPHY WITH THE AGENTS OF PSITTACOSIS AND TRACHOMA. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:912–914. doi: 10.3181/00379727-113-28529. [DOI] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J. The intracellular life of Chlamydia. Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- Schwartzman J. D., Pfefferkorn E. R. Pyrimidine synthesis by intracellular Toxoplasma gondii. J Parasitol. 1981 Apr;67(2):150–158. [PubMed] [Google Scholar]
- Spiegelman S., Sawyer R., Nayak R., Ritzi E., Stolfi R., Martin D. Improving the anti-tumor activity of 5-fluorouracil by increasing its incorporation into RNA via metabolic modulation. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4966–4970. doi: 10.1073/pnas.77.8.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Ribonucleotide reductases. Adv Enzymol Relat Areas Mol Biol. 1990;63:349–419. doi: 10.1002/9780470123096.ch6. [DOI] [PubMed] [Google Scholar]
- Tipples G., McClarty G. Isolation and initial characterization of a series of Chlamydia trachomatis isolates selected for hydroxyurea resistance by a stepwise procedure. J Bacteriol. 1991 Aug;173(16):4932–4940. doi: 10.1128/jb.173.16.4932-4940.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


