Abstract
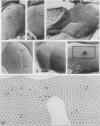
Thin sections, freeze-etched, and negatively stained preparations of Methanocorpusculum sinense cells reveal a highly lobed cell structure with a hexagonally arranged surface layer (S layer). Digital image processing of negatively stained envelope fragments show that the S layer forms a porous but strongly interconnected network. Since the S layer is the exclusive cell envelope component outside the cytoplasmic membrane it must have a cell shape determining and maintaining function. Although lattice faults such as disclinations and dislocations are a geometrical necessity on the surface of a closed protein crystal, our data indicate that they also play important roles as sites for the incorporation of new morphological units, in the formation of the lobed cell structure, and in the cell division process. In freeze-etched preparations of intact cells numerous positive and negative 60 degree wedge disclinations can be detected which form pentagons and heptagons in the hexagonal array. Complementary pairs of pentagons and heptagons are the termination points of edge dislocations. They can be expected to function both as sites for incorporation of new morphological units into the lattice and as initiation points for the cell division process. The latter is determined by the ratio between the increase of protoplast volume and the increase in actual S-layer surface area during cell growth. We postulate that this mode of cell fission represents a common feature in lobed archaebacteria which possess an S layer as the exclusive wall component.
Full text
PDF





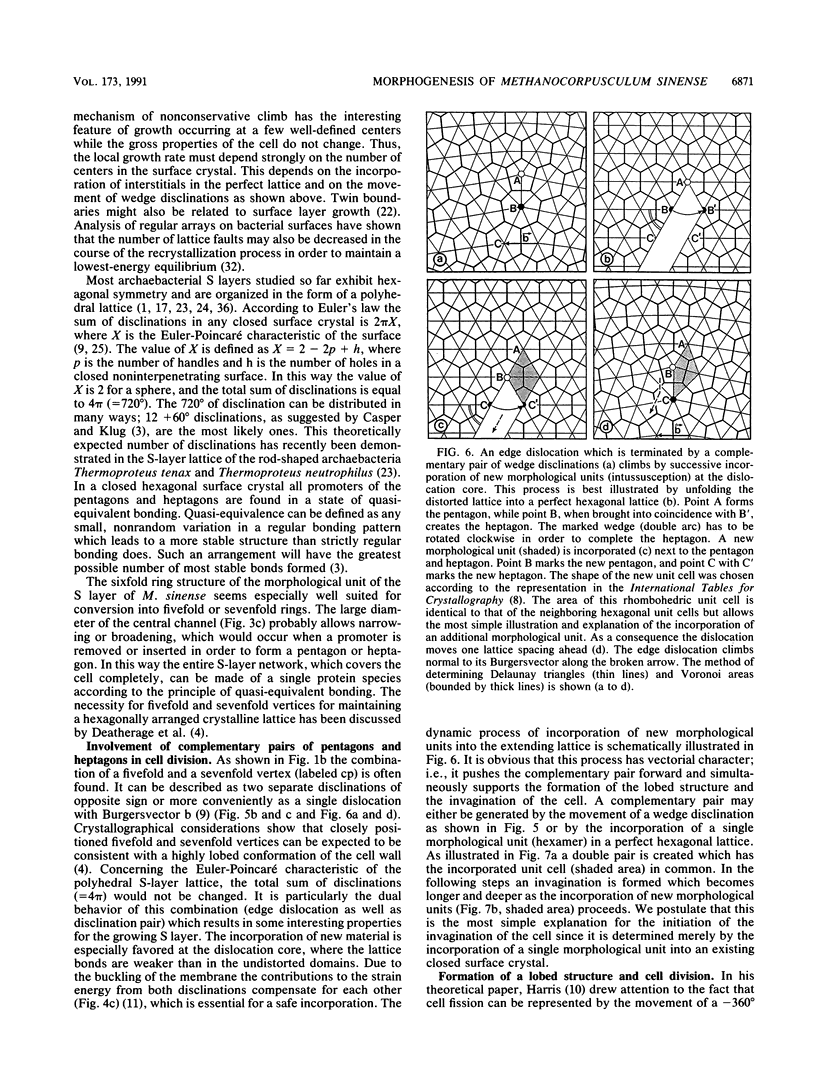
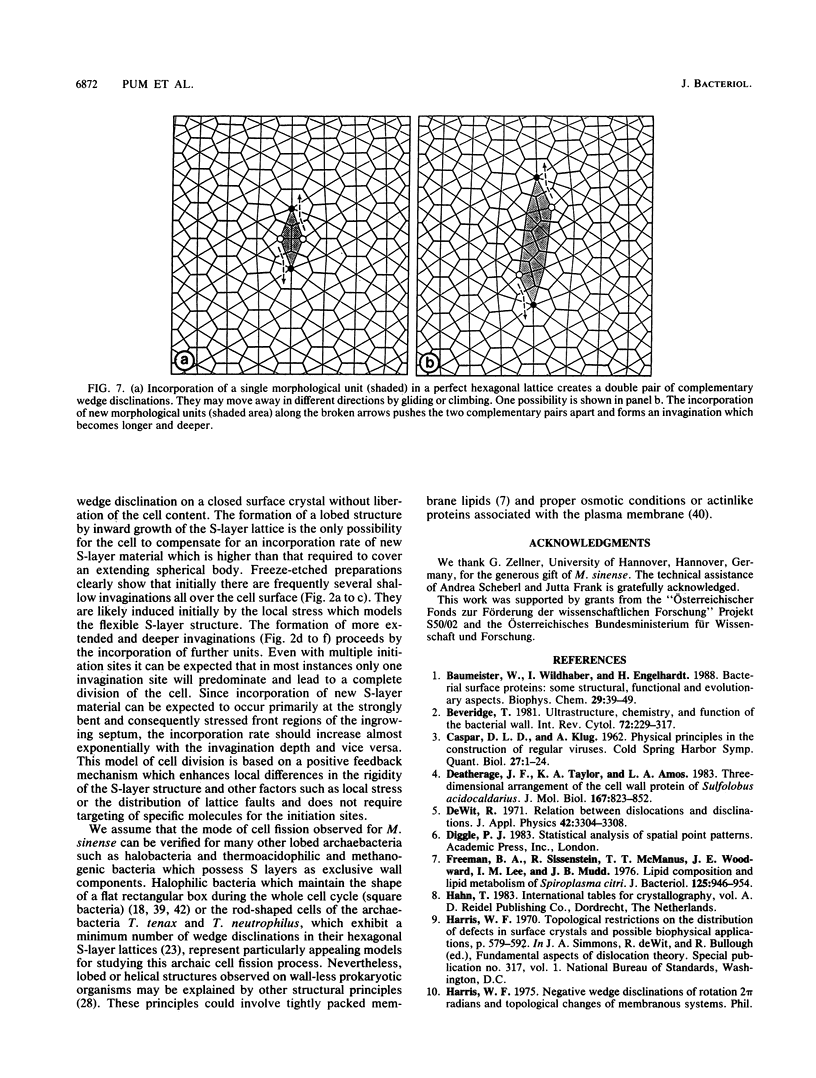

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister W., Wildhaber I., Engelhardt H. Bacterial surface proteins. Some structural, functional and evolutionary aspects. Biophys Chem. 1988 Feb;29(1-2):39–49. doi: 10.1016/0301-4622(88)87023-6. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Taylor K. A., Amos L. A. Three-dimensional arrangement of the cell wall protein of Sulfolobus acidocaldarius. J Mol Biol. 1983 Jul 15;167(4):823–848. doi: 10.1016/s0022-2836(83)80113-2. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Sissenstein R., McManus T. T., Woodward J. E., Lee I. M., Mudd J. B. Lipid composition and lipid metabolism of Spiroplasma citri. J Bacteriol. 1976 Mar;125(3):946–954. doi: 10.1128/jb.125.3.946-954.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaubert A. M., Sleytr U. B. Analysis of regular arrays of subunits on bacterial surfaces: evidence for a dynamic process of assembly. J Ultrastruct Res. 1975 Jan;50(1):103–116. doi: 10.1016/s0022-5320(75)90012-x. [DOI] [PubMed] [Google Scholar]
- Harris W. F., Scriven L. E. Function of dislocations in cell walls and membranes. Nature. 1970 Nov 28;228(5274):827–829. doi: 10.1038/228827a0. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Baumeister W. Correlation averaging of a badly distorted lattice: the surface protein of Pyrodictium occultum. J Electron Microsc Tech. 1988 Aug;9(4):413–419. doi: 10.1002/jemt.1060090407. [DOI] [PubMed] [Google Scholar]
- Hovmöller S., Sjögren A., Wang D. N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51(2):131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Cohen Y. Ultrastructure of square bacteria from a brine pool in Southern Sinai. J Bacteriol. 1982 May;150(2):851–860. doi: 10.1128/jb.150.2.851-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarro F. R., Harris W. F. Presence and function of disclinations in surface coats of unicellular organisms. Nature. 1971 Aug 6;232(5310):423–423. doi: 10.1038/232423a0. [DOI] [PubMed] [Google Scholar]
- Pum D., Ahnelt P. K., Grasl M. Iso-orientation areas in the foveal cone mosaic. Vis Neurosci. 1990 Dec;5(6):511–523. doi: 10.1017/s0952523800000687. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Stewart M. Introduction to the computer image processing of electron micrographs of two-dimensionally ordered biological structures. J Electron Microsc Tech. 1988 Aug;9(4):301–324. doi: 10.1002/jemt.1060090403. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W. Walsby's square bacterium: fine structure of an orthogonal procaryote. J Bacteriol. 1981 Oct;148(1):352–360. doi: 10.1128/jb.148.1.352-360.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Burgess J., Plaskitt K. A. Morphology and ultrastructure of helical and nonhelical strains of Spiroplasma citri. J Bacteriol. 1980 Jun;142(3):973–981. doi: 10.1128/jb.142.3.973-981.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner G., Stackebrandt E., Messner P., Tindall B. J., Conway de Macario E., Kneifel H., Sleytr U. B., Winter J. Methanocorpusculaceae fam. nov., represented by Methanocorpusculum parvum, Methanocorpusculum sinense spec. nov. and Methanocorpusculum bavaricum spec. nov. Arch Microbiol. 1989;151(5):381–390. doi: 10.1007/BF00416595. [DOI] [PubMed] [Google Scholar]