Abstract
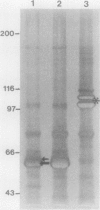
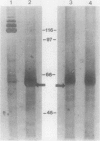
This study examined the distribution of the major outer sheath proteins (MOSP) in several Treponema denticola strains and reports the isolation of a 64-kDa protein from the outer sheath of human clinical isolate T. denticola GM-1. The outer sheath was isolated by freeze-thaw procedures, and the distribution of outer sheath proteins was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). T. denticola GM-1, MS25, SR-5, and three low-passage clinical isolates possessed an MOSP with a relative molecular mass of 60 to 64 kDa. This MOSP was absent in T. denticola ATCC 35404 (TD-4) and clinical isolate SR-4. The latter possessed an MOSP of 58 kDa. 125I labeling revealed both MOSP to be dissociated forms of higher-molecular-mass oligomeric units between 116 and 162 kDa. Two-dimensional SDS-PAGE confirmed the modifiability of these MOSP. Isoelectric focusing of the 64-kDa MOSP indicated a pI of 6.7. Immunoblots with antiserum to GM-1 whole cells revealed the 64-kDa protein to be immunogenic and not cross-reactive with the MOSP of TD-4 or SR-4, and monospecific antibody to the 64-kDa protein recognized common epitopes on the high-molecular-weight oligomeric protein. These antibodies did not react with any component of TD-4 whole cells in immunoblots or in immunogold electron microscopy. Fab fragments inhibited the adherence of T. denticola GM-1 to human gingival fibroblasts by 78% (1:1,600; 0.72 micrograms of protein per ml), while TD-4 adherence was not inhibited. Amino acid analysis revealed a slightly acidic protein, devoid of cysteine, with 36% hydrophobic residues. Cyanogen bromide fragmentation of the 64-kDa protein revealed that a 42-kDa fragment contained a T-L-D-L-A-L-D segment which was 100% homologous with an integrin alpha subunit of a human leukocyte adhesion glycoprotein p 150,95.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Heat-modifiable envelope proteins of Bordetella pertussis. Infect Immun. 1986 Oct;54(1):109–117. doi: 10.1128/iai.54.1.109-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E. Role of fibronectin in the pathogenesis of syphilis. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S372–S385. doi: 10.1093/clinids/9.supplement_4.s372. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976 Nov;128(2):616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brause B. D., Roberts R. B. Attachment of virulent Treponema pallidum to human mononuclear phagocytes. Br J Vener Dis. 1978 Aug;54(4):218–224. doi: 10.1136/sti.54.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Hakomori S. Isolation of galactoprotein a from hamster embryo fibroblasts and characterization of the carbohydrate unit. Biochemistry. 1979 Feb 20;18(4):730–738. doi: 10.1021/bi00571a027. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., DeOgny L., Slaughter C., Radolf J. D., Norgard M. V. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989 Sep;57(9):2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimasoni G., McBride B. C. Adherence of Treponema denticola to modified hydroxyapatite. J Dent Res. 1987 Dec;66(12):1727–1729. doi: 10.1177/00220345870660120601. [DOI] [PubMed] [Google Scholar]
- Cockayne A., Sanger R., Ivic A., Strugnell R. A., MacDougall J. H., Russell R. R., Penn C. W. Antigenic and structural analysis of Treponema denticola. J Gen Microbiol. 1989 Dec;135(12):3209–3218. doi: 10.1099/00221287-135-12-3209. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Garcia-Aguilar J., Springer T. A. Genomic structure of an integrin alpha subunit, the leukocyte p150,95 molecule. J Biol Chem. 1990 Feb 15;265(5):2782–2788. [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990 Dec;58(12):3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Finn M. A., Jenkin H. M. Cytopathic effects of Leptospira serotypes patoc and canicola in three kidney cell culture systems. Am J Vet Res. 1973 May;34(5):669–672. [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Hakomori S. Carbohydrate structure of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5451–5457. [PubMed] [Google Scholar]
- Fullmer C. S. Identification of cysteine-containing peptides in protein digests by high-performance liquid chromatography. Anal Biochem. 1984 Nov 1;142(2):336–339. doi: 10.1016/0003-2697(84)90473-1. [DOI] [PubMed] [Google Scholar]
- Fumarola D., Cedola M. C., Guanti G., Matsuura A., Uede T., Jirillo E. Adherence of Lyme disease spirochetes to rat lymphocytes. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):146–150. doi: 10.1016/s0176-6724(86)80117-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Fernandez-Villar B., Benach J. L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989 Sep;160(3):497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984 Jul;159(1):107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Bramanti T. E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2(2):177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Chamberlain N. R., Orth K., Moomaw C. R., Zhang L. Q., Slaughter C. A., Radolf J. D., Sell S., Norgard M. V. Sequence analysis of the 47-kilodalton major integral membrane immunogen of Treponema pallidum. Infect Immun. 1989 Jan;57(1):196–203. doi: 10.1128/iai.57.1.196-203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jacob E., Nauman R. K. Common antigens of Treponema denticola: chemical, physical, and serological characterization. Infect Immun. 1982 Aug;37(2):474–480. doi: 10.1128/iai.37.2.474-480.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G. D., Culp D. J., Jahnke M. R. Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal Biochem. 1990 Mar;185(2):324–330. doi: 10.1016/0003-2697(90)90302-p. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp D. R., Atkinson J. P., Shreffler D. C. Genetic variation in glycosylation of the fourth component of murine complement. Association with hemolytic activity. J Biol Chem. 1982 Jul 10;257(13):7330–7335. [PubMed] [Google Scholar]
- Kennell W., Holt S. C. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol Immunol. 1990 Jun;5(3):121–130. doi: 10.1111/j.1399-302x.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Parr T. R., Jr, Angus B. L., Hancock R. E., Ghiorse W. C., Greenberg E. P. Isolation of the outer membrane and characterization of the major outer membrane protein from Spirochaeta aurantia. J Bacteriol. 1987 Jan;169(1):172–179. doi: 10.1128/jb.169.1.172-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamont R. J., Rosan B., Baker C. T., Nelson G. M. Characterization of an adhesion antigen of Streptococcus sanguis G9B. Infect Immun. 1988 Sep;56(9):2417–2423. doi: 10.1128/iai.56.9.2417-2423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Masuda K., Kawata T. Isolation, properties, and reassembly of outer sheath carrying a polygonal array from an oral treponeme. J Bacteriol. 1982 Jun;150(3):1405–1413. doi: 10.1128/jb.150.3.1405-1413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. M., Witholt B. K88-mediated binding of Escherichia coli outer membrane fragments to porcine intestinal epithelial cell brush borders. Infect Immun. 1981 Jan;31(1):42–51. doi: 10.1128/iai.31.1.42-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. G., Wilson R. B. IN VIVO AND IN VITRO OBSERVATIONS OF LEPTOSPIRA POMONA BY ELECTRON MICROSCOPY. J Bacteriol. 1962 Sep;84(3):569–576. doi: 10.1128/jb.84.3.569-576.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. G., Smith R., Lehner T. Recognition of carbohydrate and protein epitopes by monoclonal antibodies to a cell wall antigen from Streptococcus mutans. Infect Immun. 1987 Mar;55(3):810–815. doi: 10.1128/iai.55.3.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Noji S., Kokeguchi S., Kato K., Kurihara H., Murayama Y., Taniguchi S. Molecular cloning and sequence analysis of antigen gene tdpA of Treponema denticola. Infect Immun. 1991 Jun;59(6):1941–1947. doi: 10.1128/iai.59.6.1941-1947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., Ganeshkumar N., Song M., McBride B. C. Identification and preliminary characterization of a Streptococcus sanguis fibrillar glycoprotein. J Bacteriol. 1987 Jan;169(1):164–171. doi: 10.1128/jb.169.1.164-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Chamberlain N. R., Swancutt M. A., Goldberg M. S. Cloning and expression of the major 47-kilodalton surface immunogen of Treponema pallidum in Escherichia coli. Infect Immun. 1986 Nov;54(2):500–506. doi: 10.1128/iai.54.2.500-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B. M., Sell S., Schell R. F. Treponema pallidum attachment to surface and matrix proteins of cultured rabbit epithelial cells. J Infect Dis. 1987 Apr;155(4):742–748. doi: 10.1093/infdis/155.4.742. [DOI] [PubMed] [Google Scholar]
- Swanson A. F., Kuo C. C. Evidence that the major outer membrane protein of Chlamydia trachomatis is glycosylated. Infect Immun. 1991 Jun;59(6):2120–2125. doi: 10.1128/iai.59.6.2120-2125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin tetrapeptide is target for syphilis spirochete cytadherence. J Exp Med. 1985 Nov 1;162(5):1715–1719. doi: 10.1084/jem.162.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Putative Treponema pallidum cytadhesins share a common functional domain. Infect Immun. 1985 Sep;49(3):833–835. doi: 10.1128/iai.49.3.833-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Higbie L. M. In vitro association of leptospires with host cells. Infect Immun. 1990 Mar;58(3):581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto M., Niikura M., Ono E., Kida H., Yanagawa R. Leptospiral attachment to cultured cells. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):268–274. doi: 10.1016/s0176-6724(84)80044-9. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Namikawa I., Suido H., Asai S. A major antigen on the outer envelope of a human oral spirochete, Treponema denticola. Infect Immun. 1989 Aug;57(8):2470–2474. doi: 10.1128/iai.57.8.2470-2474.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh T., Faine S., Adler B. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J Med Microbiol. 1984 Aug;18(1):73–85. doi: 10.1099/00222615-18-1-73. [DOI] [PubMed] [Google Scholar]
- Walfield A. M., Roche E. S., Zounes M. C., Kirkpatrick H., Wild M. A., Textor G., Tsai P. K., Richardson C. Primary structure of an oligomeric antigen of Treponema pallidum. Infect Immun. 1989 Feb;57(2):633–635. doi: 10.1128/iai.57.2.633-635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]