Abstract
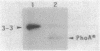
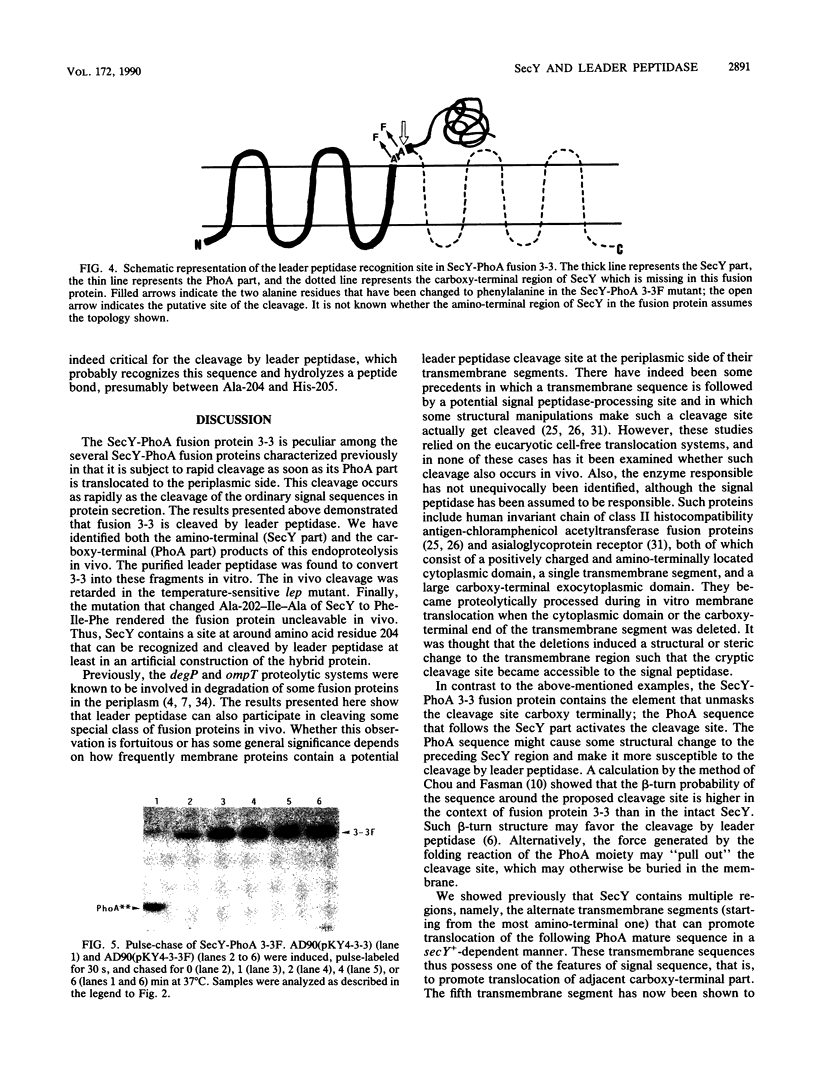
SecY is an Escherichia coli integral membrane protein required for efficient translocation of other proteins across the cytoplasmic membrane; it is embedded in this membrane by the 10 transmembrane segments. Among several SecY-alkaline phosphatase (PhoA) fusion proteins that we constructed previously, SecY-PhoA fusion 3-3, in which PhoA is fused to the third periplasmic region of SecY just after the fifth transmembrane segment, was found to be subject to rapid proteolytic processing in vivo. Both the SecY and PhoA products of this cleavage have been identified immunologically. In contrast, cleavage of SecY-PhoA 3-3 was barely observed in a lep mutant with a temperature-sensitive leader peptidase. The full-length fusion protein accumulated in this mutant was cleaved in vitro by the purified leader peptidase. A sequence Ala-202-Ile-Ala located near the proposed interface between transmembrane segment 5 and periplasmic domain 3 of SecY was found to be responsible for the recognition and cleavage by the leader peptidase, since a mutated fusion protein with Phe-Ile-Phe at this position was no longer cleaved even in the wild-type cells. These results indicate that SecY contains a potential leader peptidase cleavage site that undergoes cleavage if the PhoA sequence is attached carboxy terminally. Thus, transmembrane segment 5 of SecY can fulfill both of the two important functions of the signal peptide, translocation and cleavage, although the latter function is cryptic in the normal SecY protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Export of Escherichia coli alkaline phosphatase attached to an integral membrane protein, SecY. J Biol Chem. 1989 Jan 5;264(1):437–442. [PubMed] [Google Scholar]
- Akiyama Y., Ito K. The SecY membrane component of the bacterial protein export machinery: analysis by new electrophoretic methods for integral membrane proteins. EMBO J. 1985 Dec 1;4(12):3351–3356. doi: 10.1002/j.1460-2075.1985.tb04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx F., Georgiou G. In vivo degradation of secreted fusion proteins by the Escherichia coli outer membrane protease OmpT. J Bacteriol. 1990 Jan;172(1):491–494. doi: 10.1128/jb.172.1.491-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lazdunski C., Howard S. P. The acylated precursor form of the colicin A lysis protein is a natural substrate of the DegP protease. J Bacteriol. 1989 Nov;171(11):6316–6322. doi: 10.1128/jb.171.11.6316-6322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J Biol Chem. 1985 Dec 15;260(29):15925–15931. [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Sako T. Inhibition and resumption of processing of the staphylokinase in some Escherichia coli prlA suppressor mutants. J Biol Chem. 1988 Dec 15;263(35):19077–19082. [PubMed] [Google Scholar]
- Inada T., Court D. L., Ito K., Nakamura Y. Conditionally lethal amber mutations in the leader peptidase gene of Escherichia coli. J Bacteriol. 1989 Jan;171(1):585–587. doi: 10.1128/jb.171.1.585-587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Ito K. Identification of the secY (prlA) gene product involved in protein export in Escherichia coli. Mol Gen Genet. 1984;197(2):204–208. doi: 10.1007/BF00330964. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Wickner W. Conserved residues of the leader peptide are essential for cleavage by leader peptidase. J Biol Chem. 1985 Dec 15;260(29):15914–15918. [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Nault A. K. Characterization of the Escherichia coli protein-export gene secB. Gene. 1989 Jan 30;75(1):167–175. doi: 10.1016/0378-1119(89)90393-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal and membrane anchor functions overlap in the type II membrane protein I gamma CAT. J Cell Biol. 1988 Jun;106(6):1813–1820. doi: 10.1083/jcb.106.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 1986 Sep 26;46(7):1103–1112. doi: 10.1016/0092-8674(86)90710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Schmid S. R., Spiess M. Deletion of the amino-terminal domain of asialoglycoprotein receptor H1 allows cleavage of the internal signal sequence. J Biol Chem. 1988 Nov 15;263(32):16886–16891. [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]