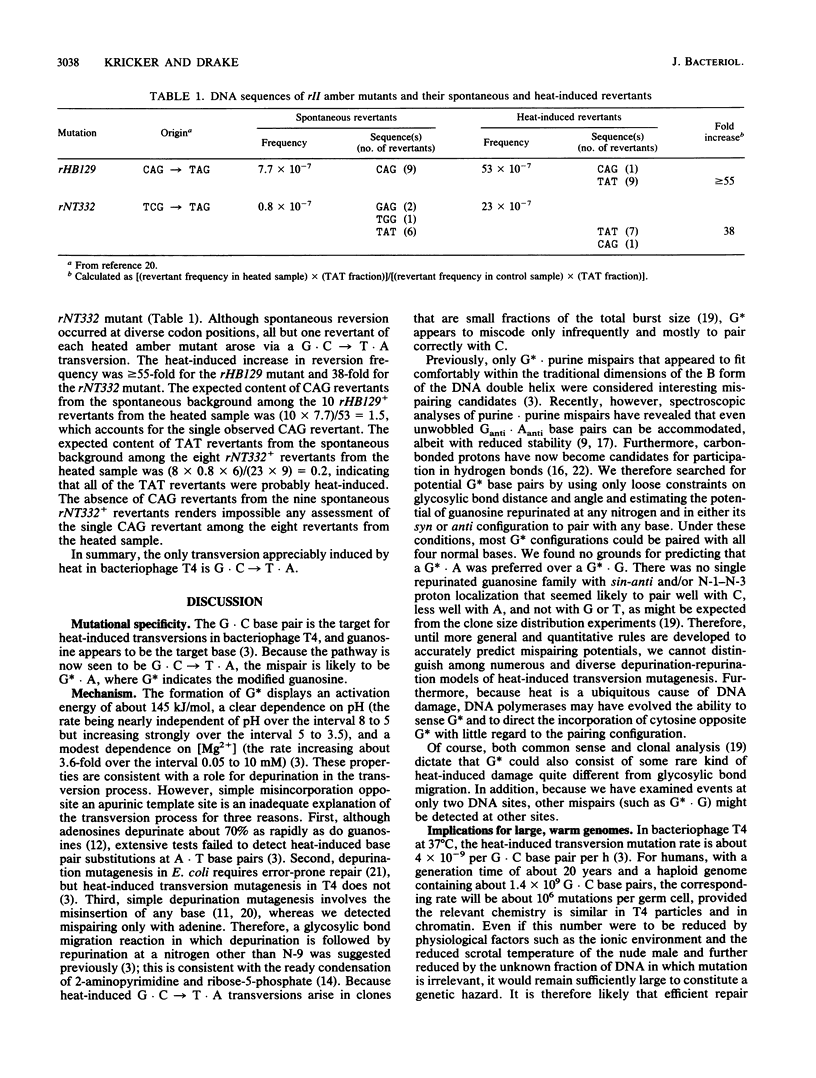
Abstract
Extracellular nonreplicating bacteriophage T4 particles accumulate mutations as functions of temperature, time, pH, and ionic environment via two mechanisms: 5-hydroxymethylcytidine deamination produces G.C----A.T transitions while a guanosine modification produces transversions. Neither frameshift mutations nor mutations at A.T base pairs are appreciably induced. We now show that heat induces G.C----T.A transversions which we suggest may arise via a G*.A mispair, in which G* is a modified guanosine that has experienced a glycosylic bond migration. The rate of this reaction at 37 degrees C is sufficient to present a genetic hazard, particularly to large genomes; thus, the lesion is probably efficiently repaired in cellular genomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au K. G., Clark S., Miller J. H., Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H., Bingham P. M., Drake J. W. Heat mutagenesis in bacteriophage T4: the transition pathway. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1269–1273. doi: 10.1073/pnas.73.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Baltz R. H., Ripley L. S., Drake J. W. Heat mutagenesis in bacteriophage T4: the transversion pathway. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4159–4163. doi: 10.1073/pnas.73.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkling M. A., Drake J. W. Isolation and characterization of conditional alleles of bacteriophage T4 genes uvsX and uvsY. Genetics. 1984 Aug;107(4):505–523. doi: 10.1093/genetics/107.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Drake J. W. Heteroduplex heterozygotes in bacteriophage T4 involving mutations of various dimensions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):506–512. doi: 10.1073/pnas.55.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Characteristics of mutations appearing spontaneously in extracellular particles of bacteriophage T4. Genetics. 1967 Mar;55(3):387–398. doi: 10.1093/genetics/55.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L. S., Chandrasegaran S., Pulford S. M., Miller P. S. Detection of a guanine X adenine base pair in a decadeoxyribonucleotide by proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4263–4265. doi: 10.1073/pnas.80.14.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricker M. C., Tindall K. R. Direct sequencing of bacteriophage T4 DNA with a thermostable DNA polymerase. Gene. 1989 Dec 21;85(1):199–204. doi: 10.1016/0378-1119(89)90481-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Lu A. L., Chang D. Y. A novel nucleotide excision repair for the conversion of an A/G mismatch to C/G base pair in E. coli. Cell. 1988 Sep 9;54(6):805–812. doi: 10.1016/s0092-8674(88)91109-9. [DOI] [PubMed] [Google Scholar]
- Mace D. C. Formation of novel nucleosides from free base and sugar phosphate: aqueous reaction of 2-aminopyrimidine and ribose-5-phosphate. Biochem Biophys Res Commun. 1983 Nov 30;117(1):93–98. doi: 10.1016/0006-291x(83)91545-0. [DOI] [PubMed] [Google Scholar]
- Nghiem Y., Cabrera M., Cupples C. G., Miller J. H. The mutY gene: a mutator locus in Escherichia coli that generates G.C----T.A transversions. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein R. L., Fresco J. R. Correlation of crystallographically determined and computationally predicted hydrogen-bonded pairing configurations of nucleic acid bases. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5171–5175. doi: 10.1073/pnas.80.17.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Ikuta S., Itakura K. Dynamics of DNA duplexes containing internal G.T, G.A, A.C, and T.C pairs: hydrogen exchange at and adjacent to mismatch sites. Fed Proc. 1984 Aug;43(11):2663–2670. [PubMed] [Google Scholar]
- Pribnow D., Sigurdson D. C., Gold L., Singer B. S., Napoli C., Brosius J., Dull T. J., Noller H. F. rII cistrons of bacteriophage T4. DNA sequence around the intercistronic divide and positions of genetic landmarks. J Mol Biol. 1981 Jul 5;149(3):337–376. doi: 10.1016/0022-2836(81)90477-0. [DOI] [PubMed] [Google Scholar]
- Ripley L. S. Estimation of in-vivo miscoding rates. J Mol Biol. 1988 Jul 5;202(1):17–34. doi: 10.1016/0022-2836(88)90514-1. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]



