Abstract
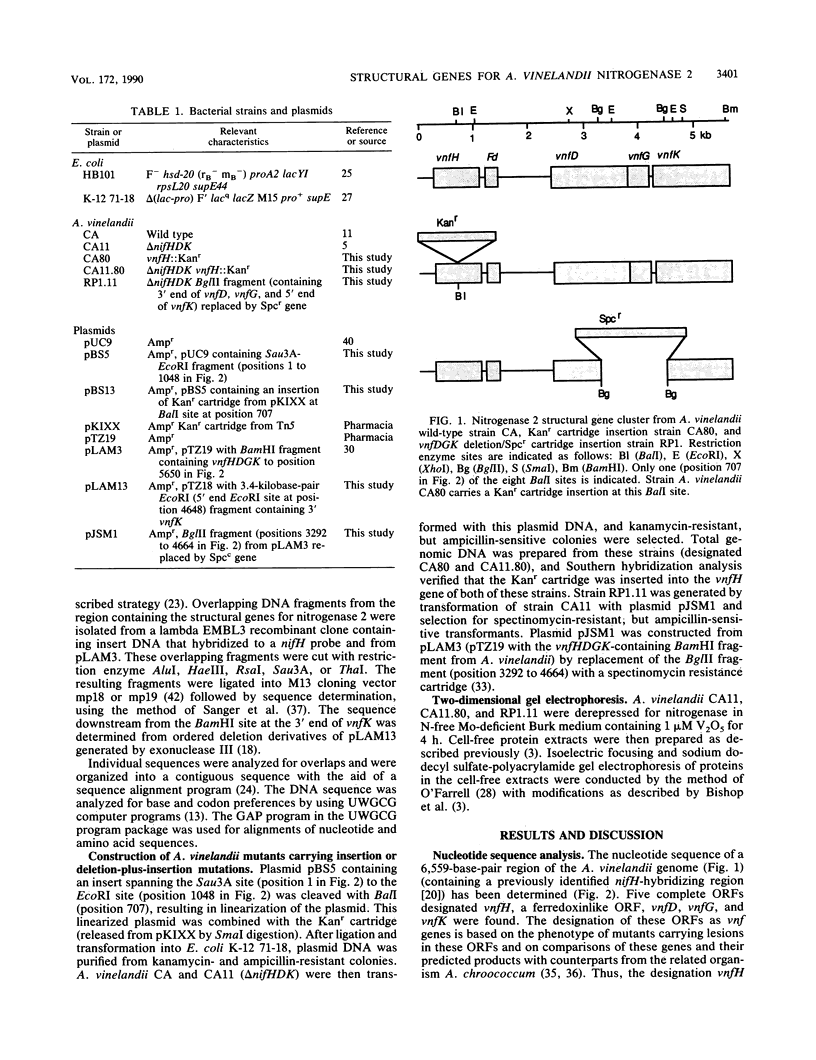
The nucleotide sequence (6,559 base pairs) of the genomic region containing the structural genes for nitrogenase 2 (V nitrogenase) from Azotobacter vinelandii was determined. The open reading frames present in this region are organized into two transcriptional units. One contains vnfH (encoding dinitrogenase reductase 2) and a ferredoxinlike open reading frame (Fd). The second one includes vnfD (encoding the alpha subunit of dinitrogenase 2), vnfG (encoding a product similar to the delta subunit of dinitrogenase 2 from A. chroococcum), and vnfK (encoding the beta subunit of dinitrogenase 2). The 5'-flanking regions of vnfH and vnfD contain sequences similar to ntrA-dependent promoters. This gene arrangement allows independent expression of vnfH-Fd and vnfDGK. Mutant strains (CA80 and CA11.80) carrying an insertion in vnfH are still able to synthesize the alpha and beta subunits of dinitrogenase 2 when grown in N-free, Mo-deficient, V-containing medium. A strain (RP1.11) carrying a deletion-plus-insertion mutation in the vnfDGK region produced only dinitrogenase reductase 2.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Hawkins M. E., Eady R. R. Nitrogen fixation in molybdenum-deficient continuous culture by a strain of Azotobacter vinelandii carrying a deletion of the structural genes for nitrogenase (nifHDK). Biochem J. 1986 Sep 1;238(2):437–442. doi: 10.1042/bj2380437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Expression of an alternative nitrogen fixation system in Azotobacter vinelandii. J Bacteriol. 1982 Jun;150(3):1244–1251. doi: 10.1128/jb.150.3.1244-1251.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Premakumar R., Dean D. R., Jacobson M. R., Chisnell J. R., Rizzo T. M., Kopczynski J. Nitrogen Fixation by Azotobacter vinelandii Strains Having Deletions in Structural Genes for Nitrogenase. Science. 1986 Apr 4;232(4746):92–94. doi: 10.1126/science.232.4746.92. [DOI] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Premakumar R., Bishop P. E. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol. 1988 Jan;170(1):27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. J., Case E. E., Morningstar J. E., Dzeda M. F., Mauterer L. A. Isolation of a new vanadium-containing nitrogenase from Azotobacter vinelandii. Biochemistry. 1986 Nov 18;25(23):7251–7255. doi: 10.1021/bi00371a001. [DOI] [PubMed] [Google Scholar]
- Hales B. J., Langosch D. J., Case E. E. Isolation and characterization of a second nitrogenase Fe-protein from Azotobacter vinelandii. J Biol Chem. 1986 Nov 15;261(32):15301–15306. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. R., Brigle K. E., Bennett L. T., Setterquist R. A., Wilson M. S., Cash V. L., Beynon J., Newton W. E., Dean D. R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Bacterial alternative nitrogen fixation systems. Crit Rev Microbiol. 1988;16(1):1–14. doi: 10.3109/10408418809104465. [DOI] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Nucleotide sequence and genetic analysis of the nifB-nifQ region from Azotobacter vinelandii. J Bacteriol. 1988 Apr;170(4):1475–1487. doi: 10.1128/jb.170.4.1475-1487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Premakumar R., Wolfinger E. D., Bishop P. E. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1075–1086. doi: 10.1128/jb.171.2.1075-1086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Mackenzie J. M., Jr, Dougherty W. G. Assembly of overlapping DNA sequences by a program written in BASIC for 64K CP/M and MS-DOS IBM-compatible microcomputers. Nucleic Acids Res. 1986 Jan 10;14(1):517–527. doi: 10.1093/nar/14.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau R. N., Mitchenall L. A., Robson R. L. Genetic evidence for an Azotobacter vinelandii nitrogenase lacking molybdenum and vanadium. J Bacteriol. 1989 Jan;171(1):124–129. doi: 10.1128/jb.171.1.124-129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Raina R., Reddy M. A., Ghosal D., Das H. K. Characterization of the gene for the Fe-protein of the vanadium dependent alternative nitrogenase of Azotobacter vinelandii and construction of a Tn5 mutant. Mol Gen Genet. 1988 Sep;214(1):121–127. doi: 10.1007/BF00340189. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Woodley P. R., Pau R. N., Eady R. R. Structural genes for the vanadium nitrogenase from Azotobacter chroococcum. EMBO J. 1989 Apr;8(4):1217–1224. doi: 10.1002/j.1460-2075.1989.tb03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R., Woodley P., Jones R. Second gene (nifH*) coding for a nitrogenase iron protein in Azotobacter chroococcum is adjacent to a gene coding for a ferredoxin-like protein. EMBO J. 1986 Jun;5(6):1159–1163. doi: 10.1002/j.1460-2075.1986.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Chen J. S., Johnson J. L. The presence of five nifH-like sequences in Clostridium pasteurianum: sequence divergence and transcription properties. Nucleic Acids Res. 1988 Jan 25;16(2):439–454. doi: 10.1093/nar/16.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]