Abstract
The 90-kb virulence plasmid of Salmonella typhimurium is necessary for invasion beyond the Peyer's patches to the mesenteric lymph nodes and spleens of orally inoculated mice. Two Tn5 insertions located on the left side of a previously identified 14-kb virulence region (P. A. Gulig and R. Curtiss III, Infect. Immun. 58:3262-3271, 1988) and mapping 272 bp from each other exhibited opposite effects on splenic infection of mice after oral inoculation. spvR23::Tn5 decreased splenic infection by 1,000-fold, whereas a spv-14::Tn5 mutant outcompeted wild-type S. typhimurium for splenic infection by 27-fold in mice fed mixtures of mutated and wild-type S. typhimurium. spvR23::Tn5 was complemented by a virulence plasmid subclone with an insert sequence encoding only an 891-bp open reading frame specifying a 33,000-molecular-weight protein. The amino acid sequence of this open reading frame had significant homology to members of the LysR family of positive regulatory proteins; thus, the gene was named spvR (salmonella plasmid virulence). To examine the possible regulatory effects of spvR on other virulence genes, we constructed a lacZ operon fusion in a downstream virulence gene, spvB. When spvR subcloned behind the lac promoter was provided on a separate plasmid in trans to the spvB-lacZ operon fusion, transcription of spvB increased 15-fold. spv-14::Tn5, which conferred a competitive advantage to S. typhimurium, increased the expression of a spvR-lacZ operon fusion in cis. spvR is therefore a positive regulator of spvB and an essential virulence gene of S. typhimurium. As opposed to having spvR subcloned behind the lac promoter, the wild-type spvR gene present on the virulence plasmid did not function to positively regulate spvB-lacZ in trans when salmonellae were grown to the log phase in L broth, suggesting that this regulatory system is activated in vivo during infection.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird G. D., Manning E. J., Jones P. W. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J Gen Microbiol. 1985 Jul;131(7):1815–1823. doi: 10.1099/00221287-131-7-1815. [DOI] [PubMed] [Google Scholar]
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Byerly K. A., Urbanowski M. L., Stauffer G. V. Escherichia coli metR mutants that produce a MetR activator protein with an altered homocysteine response. J Bacteriol. 1990 Jun;172(6):2839–2843. doi: 10.1128/jb.172.6.2839-2843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Y., Maxon M. E., Redfield B., Glass R., Brot N., Weissbach H. Methionine synthesis in Escherichia coli: effect of the MetR protein on metE and metH expression. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4407–4411. doi: 10.1073/pnas.86.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Y., Redfield B., Maxon M., Weissbach H., Brot N. The effect of homocysteine on MetR regulation of metE, metR and metH expression in vitro. Biochem Biophys Res Commun. 1989 Aug 30;163(1):79–83. doi: 10.1016/0006-291x(89)92101-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. A general method for fusion of the Escherichia coli lacZ gene to chromosomal genes in Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2953–2966. doi: 10.1099/00221287-132-11-2953. [DOI] [PubMed] [Google Scholar]
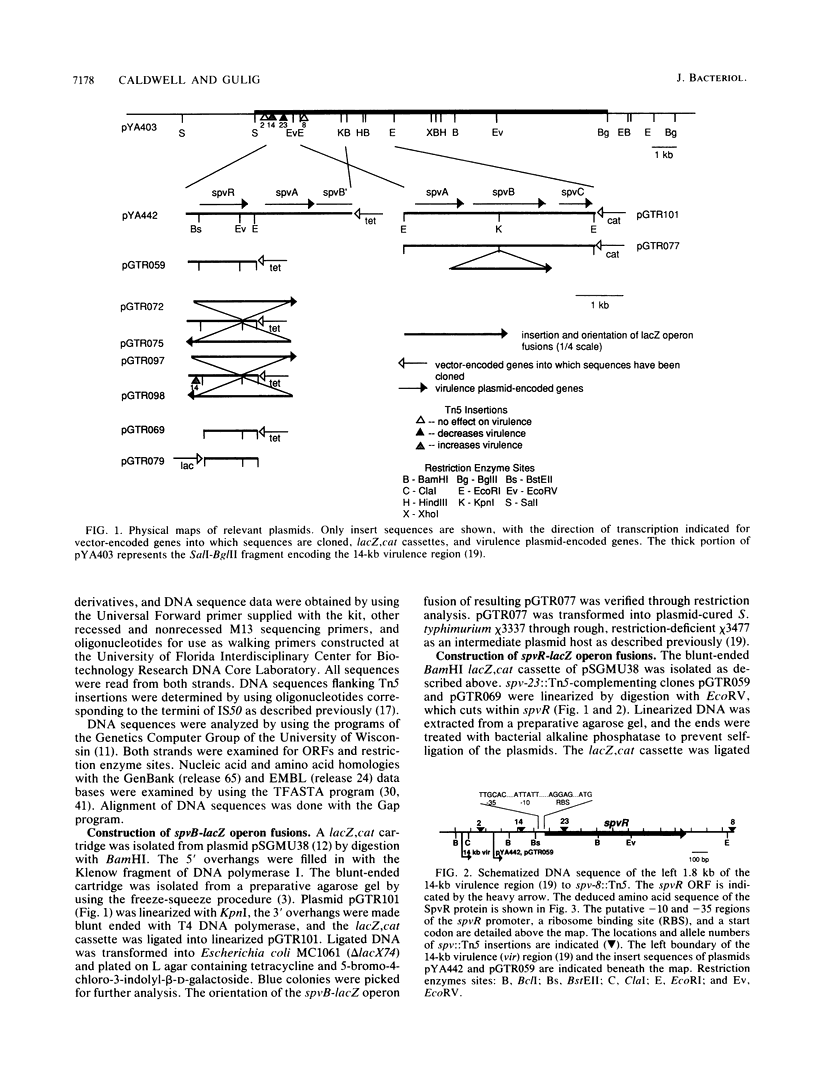
- Gulig P. A., Chiodo V. A. Genetic and DNA sequence analysis of the Salmonella typhimurium virulence plasmid gene encoding the 28,000-molecular-weight protein. Infect Immun. 1990 Aug;58(8):2651–2658. doi: 10.1128/iai.58.8.2651-2658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Cloning and transposon insertion mutagenesis of virulence genes of the 100-kilobase plasmid of Salmonella typhimurium. Infect Immun. 1988 Dec;56(12):3262–3271. doi: 10.1128/iai.56.12.3262-3271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A. Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb Pathog. 1990 Jan;8(1):3–11. doi: 10.1016/0882-4010(90)90003-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertt B. E., Ou J., Kopecko D. J., Baron L. S., Warren R. L. Novel virulence properties of the Salmonella typhimurium virulence-associated plasmid: immune suppression and stimulation of splenomegaly. Plasmid. 1989 Jan;21(1):48–58. doi: 10.1016/0147-619x(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Tsuchimoto M., Sudo K., Terakado N., Danbara H. Identification and mapping of mba regions of the Salmonella choleraesuis virulence plasmid pKDSC50 responsible for mouse bacteremia. Microb Pathog. 1990 Jan;8(1):13–21. doi: 10.1016/0882-4010(90)90004-a. [DOI] [PubMed] [Google Scholar]
- Krause M., Harwood J., Fierer J., Guiney D. Genetic analysis of homology between the virulence plasmids of Salmonella dublin and Yersinia pseudotuberculosis. Infect Immun. 1991 May;59(5):1860–1863. doi: 10.1128/iai.59.5.1860-1863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Roudier C., Fierer J., Harwood J., Guiney D. Molecular analysis of the virulence locus of the Salmonella dublin plasmid pSDL2. Mol Microbiol. 1991 Feb;5(2):307–316. doi: 10.1111/j.1365-2958.1991.tb02111.x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lax A. J., Pullinger G. D., Baird G. D., Williamson C. M. The virulence plasmid of Salmonella dublin: detailed restriction map and analysis by transposon mutagenesis. J Gen Microbiol. 1990 Jun;136(6):1117–1123. doi: 10.1099/00221287-136-6-1117. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Matsui H., Kawahara K., Terakado N., Danbara H. Nucleotide sequence of a gene encoding a 29 kDa polypeptide in mba region of the virulence plasmid, pKDSC50, of Salmonella choleraesuis. Nucleic Acids Res. 1990 Feb 25;18(4):1055–1055. doi: 10.1093/nar/18.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Kawahara K., Terakado N., Danbara H. Nucleotide sequences of genes encoding 32 kDa and 70 kDa polypeptides in mba region of the virulence plasmid, pKDSc50, of Salmonella choleraesuis. Nucleic Acids Res. 1990 Apr 25;18(8):2181–2182. doi: 10.1093/nar/18.8.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M. E., Redfield B., Cai X. Y., Shoeman R., Fujita K., Fisher W., Stauffer G., Weissbach H., Brot N. Regulation of methionine synthesis in Escherichia coli: effect of the MetR protein on the expression of the metE and metR genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):85–89. doi: 10.1073/pnas.86.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Popoff M. Y., Durviaux S., Coynault C., Cornelis G. A new method for the physical and genetic mapping of large plasmids: application to the localisation of the virulence determinants on the 90 kb plasmid of Salmonella typhimurium. Microb Pathog. 1987 Aug;3(2):109–116. doi: 10.1016/0882-4010(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Norel F., Coynault C., Miras I., Hermant D., Popoff M. Y. Cloning and expression of plasmid DNA sequences involved in Salmonella serotype typhimurium virulence. Mol Microbiol. 1989 Jun;3(6):733–743. doi: 10.1111/j.1365-2958.1989.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Norel F., Pisano M. R., Nicoli J., Popoff M. Y. A plasmid-borne virulence region (2.8 kb) from Salmonella typhimurium contains two open reading frames. Res Microbiol. 1989 Nov-Dec;140(9):627–630. doi: 10.1016/0923-2508(89)90194-0. [DOI] [PubMed] [Google Scholar]
- Norel F., Pisano M. R., Nicoli J., Popoff M. Y. Nucleotide sequence of the plasmid-borne virulence gene mkfA encoding a 28 kDa polypeptide from Salmonella typhimurium. Res Microbiol. 1989 Mar-Apr;140(3):263–265. doi: 10.1016/0923-2508(89)90081-8. [DOI] [PubMed] [Google Scholar]
- Norel F., Pisano M. R., Nicoli J., Popoff M. Y. Nucleotide sequence of the plasmid-borne virulence gene mkfB from Salmonella typhimurium. Res Microbiol. 1989 Sep;140(7):455–457. doi: 10.1016/0923-2508(89)90066-1. [DOI] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann L. S., Stauffer G. V. Nucleotide sequence of the Salmonella typhimurium metR gene and the metR-metE control region. J Bacteriol. 1987 Sep;169(9):3932–3937. doi: 10.1128/jb.169.9.3932-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M. D., Stauffer G. V. Regulation of the Escherichia coli glyA gene by the metR gene product and homocysteine. J Bacteriol. 1989 Sep;171(9):4958–4962. doi: 10.1128/jb.171.9.4958-4962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger G. D., Baird G. D., Williamson C. M., Lax A. J. Nucleotide sequence of a plasmid gene involved in the virulence of salmonellas. Nucleic Acids Res. 1989 Oct 11;17(19):7983–7983. doi: 10.1093/nar/17.19.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Virtanen M., Mäkelä P. H. Localization by insertion mutagenesis of a virulence-associated region on the Salmonella typhimurium 96 kilobase pair plasmid. Microb Pathog. 1989 Feb;6(2):153–158. doi: 10.1016/0882-4010(89)90018-1. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Stragier P., Patte J. C. Regulation of diaminopimelate decarboxylase synthesis in Escherichia coli. III. Nucleotide sequence and regulation of the lysR gene. J Mol Biol. 1983 Aug 5;168(2):333–350. doi: 10.1016/s0022-2836(83)80022-9. [DOI] [PubMed] [Google Scholar]
- Taira S., Rhen M. Identification and genetic analysis of mkaA--a gene of the Salmonella typhimurium virulence plasmid necessary for intracellular growth. Microb Pathog. 1989 Sep;7(3):165–173. doi: 10.1016/0882-4010(89)90052-1. [DOI] [PubMed] [Google Scholar]
- Taira S., Rhen M. Molecular organization of genes constituting the virulence determinant on the Salmonella typhimurium 96 kilobase pair plasmid. FEBS Lett. 1989 Nov 6;257(2):274–278. doi: 10.1016/0014-5793(89)81551-0. [DOI] [PubMed] [Google Scholar]
- Taira S., Rhen M. Nucleotide sequence of mkaD, a virulence-associated gene of Salmonella typhimurium containing variable and constant regions. Gene. 1990 Sep 1;93(1):147–150. doi: 10.1016/0378-1119(90)90150-p. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Genetic and biochemical analysis of the MetR activator-binding site in the metE metR control region of Salmonella typhimurium. J Bacteriol. 1989 Oct;171(10):5620–5629. doi: 10.1128/jb.171.10.5620-5629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Role of homocysteine in metR-mediated activation of the metE and metH genes in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1989 Jun;171(6):3277–3281. doi: 10.1128/jb.171.6.3277-3281.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Nucleotide sequence and in vivo expression of the ilvY and ilvC genes in Escherichia coli K12. Transcription from divergent overlapping promoters. J Biol Chem. 1986 Feb 15;261(5):2441–2450. [PubMed] [Google Scholar]
- Williamson C. M., Baird G. D., Manning E. J. A common virulence region on plasmids from eleven serotypes of Salmonella. J Gen Microbiol. 1988 Apr;134(4):975–982. doi: 10.1099/00221287-134-4-975. [DOI] [PubMed] [Google Scholar]
- Williamson C. M., Pullinger G. D., Lax A. J. Identification of an essential virulence region on Salmonella plasmids. Microb Pathog. 1988 Dec;5(6):469–473. doi: 10.1016/0882-4010(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Williamson C. M., Pullinger G. D., Lax A. J. Identification of proteins expressed by the essential virulence region of the Salmonella dublin plasmid. Microb Pathog. 1990 Jul;9(1):61–66. doi: 10.1016/0882-4010(90)90041-n. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]


