Abstract
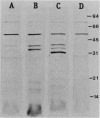
Mutants which are defective in catabolite repression control (CRC) of multiple independently regulated catabolic pathways have been previously described. The mutations were mapped at 11 min on the Pseudomonas aeruginosa chromosome and designated crc. This report describes the cloning of a gene which restores normal CRC to these Crc- mutants in trans. The gene expressing this CRC activity was subcloned on a 2-kb piece of DNA. When this 2-kb fragment was placed in a plasmid behind a phage T7 promoter and transcribed by T7 RNA polymerase, a soluble protein with a molecular weight (MW) of about 30,000 was produced in Escherichia coli. A soluble protein of identical size was overproduced in a Crc- mutant when it contained the 2-kb fragment on a multicopy plasmid. This protein could not be detected in the mutant containing the vector without the 2-kb insert or with no plasmid. When a 0.3-kb AccI fragment was removed from the crc gene and replaced with a kanamycin resistance cassette, the interrupted crc gene no longer restored CRC to the mutant, and the mutant containing the interrupted gene no longer overproduced the 30,000-MW protein. Pools of intracellular cyclic AMP and the activities of adenylate cyclase and phosphodiesterase were measured in mutant and wild-type strains with and without a plasmid containing the crc gene. No consistent differences between any strains were found in any case. These results provide original evidence for a 30,000-MW protein encoded by crc+ that is required for wild-type CRC in P. aeruginosa and confirms earlier reports that the mode of CRC is cyclic AMP independent in this bacterium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAMMAR W. J., CLARKE P. H. INDUCTION AND REPRESSION OF PSEUDOMONAS AERUGINOSA AMIDASE. J Gen Microbiol. 1964 Dec;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskey S. M., Wolff J. A., Phibbs P. V., Jr, Olsen R. H. Cloning of genes specifying carbohydrate catabolism in Pseudomonas aeruginosa and Pseudomonas putida. J Bacteriol. 1985 Jun;162(3):865–871. doi: 10.1128/jb.162.3.865-871.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Kight-Olliff L. C., Stewart G. J., Beauchamp N. F. Reversal of succinate-mediated catabolite repression of alkylsulfatase in Pseudomonas aeruginosa by 2,4-dinitrophenol and by sodium malonate. Can J Microbiol. 1978 Dec;24(12):1567–1573. doi: 10.1139/m78-251. [DOI] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. A Pseudomonas aeruginosa mutant non-derepressible for orthophosphate-regulated proteins. J Bacteriol. 1981 Aug;147(2):675–678. doi: 10.1128/jb.147.2.675-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Hamood A. N., Iglewski B. H. Expression of the Pseudomonas aeruginosa toxA positive regulatory gene (regA) in Escherichia coli. J Bacteriol. 1990 Feb;172(2):589–594. doi: 10.1128/jb.172.2.589-594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Krieg N. R., Phibbs P. V., Jr Transport and catabolism of D-fructose by Spirillum itersomii. J Bacteriol. 1974 Jan;117(1):144–150. doi: 10.1128/jb.117.1.144-150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Lory S., Strom M. S., Johnson K. Expression and secretion of the cloned Pseudomonas aeruginosa exotoxin A by Escherichia coli. J Bacteriol. 1988 Feb;170(2):714–719. doi: 10.1128/jb.170.2.714-719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. F. Transduction of Pseudomonas aeruginosa with a mutant of bacteriophage E79. J Bacteriol. 1979 Jul;139(1):137–140. doi: 10.1128/jb.139.1.137-140.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., DeBusscher G., McCombie W. R. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1982 Apr;150(1):60–69. doi: 10.1128/jb.150.1.60-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. T., Mulfinger L. M. Cyclic adenosine 3',5'-monophosphate levels in Pseudomonas putida and Pseudomonas aeruginosa during induction and carbon catabolite repression of histidase synthesis. J Bacteriol. 1981 Mar;145(3):1286–1292. doi: 10.1128/jb.145.3.1286-1292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. R., Clarke P. H. The effect of nitrogen limitation on catabolite repression of amidase, histidase and urocanase in Pseudomonas aeruginosa. J Gen Microbiol. 1976 Apr;93(2):377–387. doi: 10.1099/00221287-93-2-377. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvano M. A., Lisa T. A., Domenech C. E. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. 1989 Jan 23;85(1):81–89. doi: 10.1007/BF00223517. [DOI] [PubMed] [Google Scholar]
- Shen B. F., Tai P. C., Pritchard A. E., Vasil M. L. Nucleotide sequences and expression in Escherichia coli of the in-phase overlapping Pseudomonas aeruginosa plcR genes. J Bacteriol. 1987 Oct;169(10):4602–4607. doi: 10.1128/jb.169.10.4602-4607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. S., Hylemon P. B., Phibbs P. V., Jr Cyclic adenosine 3',5'-monophosphate levels and activities of adenylate cyclase and cyclic adenosine 3',5'-monophosphate phosphodiesterase in Pseudomonas and Bacteroides. J Bacteriol. 1977 Jan;129(1):87–96. doi: 10.1128/jb.129.1.87-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Smyth P. F., Clarke P. H. Catabolite repression of Pseudomonas aeruginosa amidase: isolation of promotor mutants. J Gen Microbiol. 1975 Sep;90(1):91–99. doi: 10.1099/00221287-90-1-91. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Purification and properties of the periplasmic glucose-binding protein of Pseudomonas aeruginosa. J Bacteriol. 1977 Aug;131(2):672–681. doi: 10.1128/jb.131.2.672-681.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Moore R. J., Krishnapillai V. Complementation analysis in Pseudomonas aeruginosa of the transfer genes of the wide host range R plasmid R18. Plasmid. 1981 Mar;5(2):202–212. doi: 10.1016/0147-619x(81)90021-4. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., MacGregor C. H., Eisenberg R. C., Phibbs P. V., Jr Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J Bacteriol. 1991 Aug;173(15):4700–4706. doi: 10.1128/jb.173.15.4700-4706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylstra G. J., Olsen R. H., Ballou D. P. Cloning, expression, and regulation of the Pseudomonas cepacia protocatechuate 3,4-dioxygenase genes. J Bacteriol. 1989 Nov;171(11):5907–5914. doi: 10.1128/jb.171.11.5907-5914.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]