Abstract
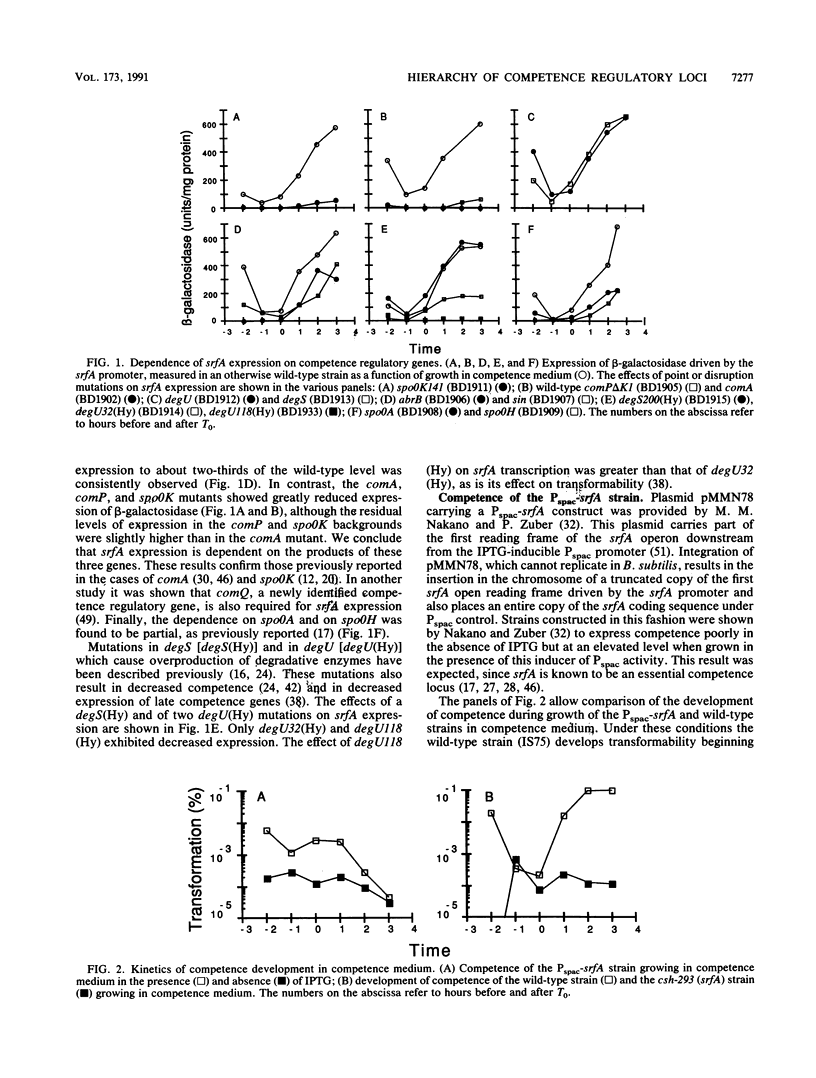
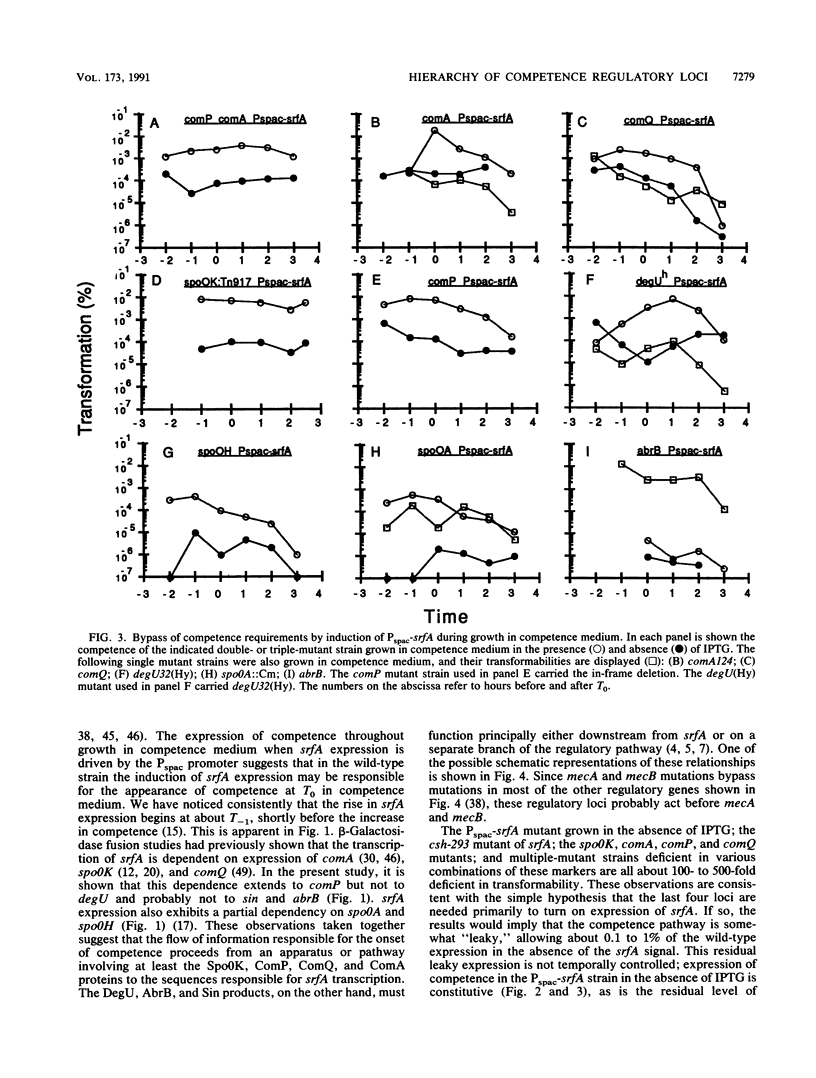
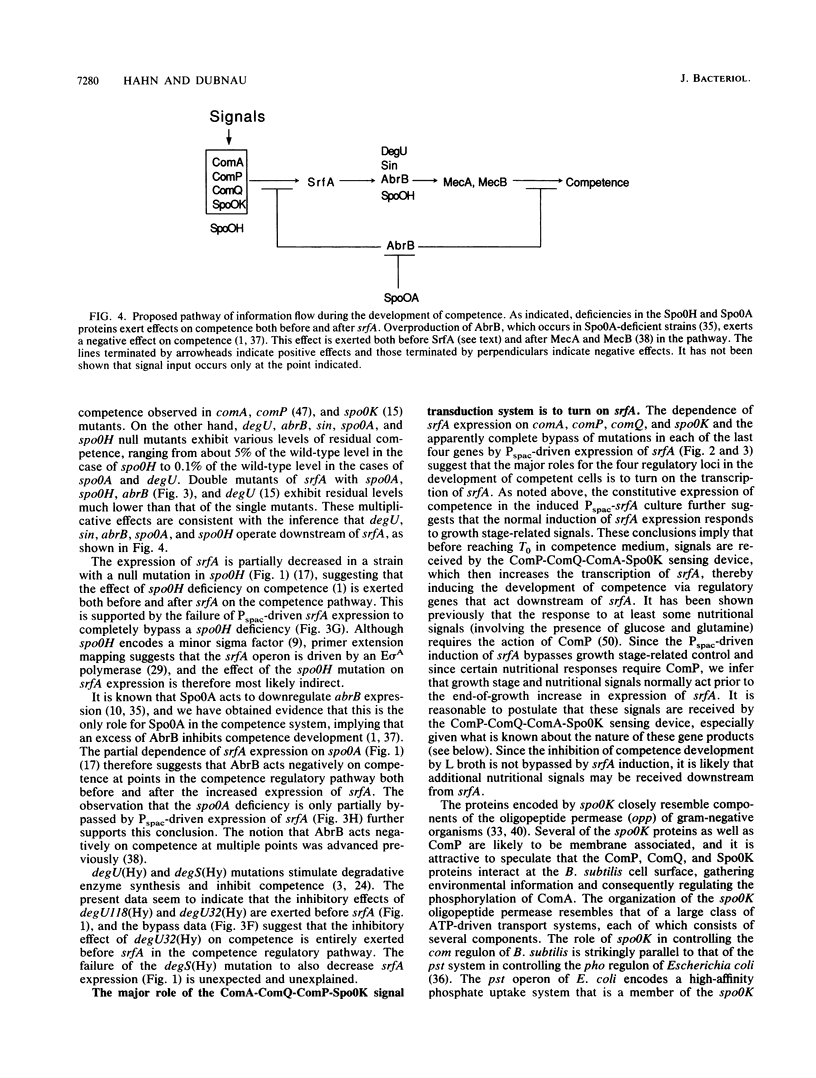
srfA is an operon needed for the development of genetic competence in Bacillus subtilis. This operon is normally expressed at a low level during growth, and its transcription increases sharply just before the transition to stationary phase. The genetic requirements for the full expression of srfA were previously examined in several laboratories and shown to include spo0A, spo0H, spo0K, comQ, and comA. In the present study these results were confirmed with an isogenic set of strains. We have also shown that comP is needed for srfA expression but that other regulatory genes required for competence (degU, sin, and abrB) are not needed for the expression of srfA. We have used the expression of srfA under control of the regulatable Pspac promoter to study the kinetics of competence development and to determine whether the genes ordinarily required for expression of srfA are needed for any additional roles during the development of competence. When expression of srfA was driven from Pspac, competence was expressed constitutively throughout growth. Furthermore, when srfA was expressed from Pspac, the spo0K, comQ, comP, and comA determinants were no longer required for the expression of competence. We conclude therefore that the multiple signals which trigger the initiation of competence development in relation to growth stage are ordinarily received prior to the increase in srfA expression. We propose that these signals are mediated by the products of spo0K, comQ, comP, and comA, resulting in the phosphorylation of ComA by ComP. This in turn would enable ComA to function as a positive transcription factor for srfA, leading to the elaboration of the srfA product(s) and the consequent initiation of competence. We also propose that this is the major, and possibly the only, role for the spo0K, comQ, comP, and comA products during competence development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Genetic analysis of oligosporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):17–27. doi: 10.1099/00221287-71-1-17. [DOI] [PubMed] [Google Scholar]
- Dahl M. K., Msadek T., Kunst F., Rapoport G. Mutational analysis of the Bacillus subtilis DegU regulator and its phosphorylation by the DegS protein kinase. J Bacteriol. 1991 Apr;173(8):2539–2547. doi: 10.1128/jb.173.8.2539-2547.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991 Sep;55(3):395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Roggiani M. Growth medium-independent genetic competence mutants of Bacillus subtilis. J Bacteriol. 1990 Jul;172(7):4048–4055. doi: 10.1128/jb.172.7.4048-4055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. The regulation of genetic competence in Bacillus subtilis. Mol Microbiol. 1991 Jan;5(1):11–18. doi: 10.1111/j.1365-2958.1991.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Weir J., Nair G., Carter L., 3rd, Moran C., Jr, Smith I. Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol. 1988 Mar;170(3):1054–1062. doi: 10.1128/jb.170.3.1054-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürbass R., Gocht M., Zuber P., Marahiel M. A. Interaction of AbrB, a transcriptional regulator from Bacillus subtilis with the promoters of the transition state-activated genes tycA and spoVG. Mol Gen Genet. 1991 Mar;225(3):347–354. doi: 10.1007/BF00261673. [DOI] [PubMed] [Google Scholar]
- Gaur N. K., Dubnau E., Smith I. Characterization of a cloned Bacillus subtilis gene that inhibits sporulation in multiple copies. J Bacteriol. 1986 Nov;168(2):860–869. doi: 10.1128/jb.168.2.860-869.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Israeli-Reches M., Dubnau D. Induction of macrolide-lincosamide-streptogramin B resistance requires ribosomes able to bind inducer. Mol Gen Genet. 1984;194(3):357–361. doi: 10.1007/BF00425544. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Yang M., Ferrari E. Localization of Bacillus subtilis sacU(Hy) mutations to two linked genes with similarities to the conserved procaryotic family of two-component signalling systems. J Bacteriol. 1988 Nov;170(11):5102–5109. doi: 10.1128/jb.170.11.5102-5109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks K. J., Healy J., Losick R., Grossman A. D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989 Aug;171(8):4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Gruber M., Venema G. Stimulation of the development of competence by culture fluids in Bacillus subtilis transformation. Biochim Biophys Acta. 1972 Mar 14;262(2):189–199. doi: 10.1016/0005-2787(72)90232-8. [DOI] [PubMed] [Google Scholar]
- Kunst F., Debarbouille M., Msadek T., Young M., Mauel C., Karamata D., Klier A., Rapoport G., Dedonder R. Deduced polypeptides encoded by the Bacillus subtilis sacU locus share homology with two-component sensor-regulator systems. J Bacteriol. 1988 Nov;170(11):5093–5101. doi: 10.1128/jb.170.11.5093-5101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S., Dubnau D. Transcriptional regulation of comC: evidence for a competence-specific transcription factor in Bacillus subtilis. J Bacteriol. 1990 Jul;172(7):4064–4071. doi: 10.1128/jb.172.7.4064-4071.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msadek T., Kunst F., Henner D., Klier A., Rapoport G., Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990 Feb;172(2):824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msadek T., Kunst F., Klier A., Rapoport G. DegS-DegU and ComP-ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J Bacteriol. 1991 Apr;173(7):2366–2377. doi: 10.1128/jb.173.7.2366-2377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K., Kawata M., Tanaka T. Isolation and phosphorylation of the Bacillus subtilis degS and degU gene products. J Biol Chem. 1990 Nov 15;265(32):20000–20006. [PubMed] [Google Scholar]
- Nakano M. M., Magnuson R., Myers A., Curry J., Grossman A. D., Zuber P. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991 Mar;173(5):1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Xia L. A., Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991 Sep;173(17):5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989 Oct;171(10):5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. The primary role of comA in establishment of the competent state in Bacillus subtilis is to activate expression of srfA. J Bacteriol. 1991 Nov;173(22):7269–7274. doi: 10.1128/jb.173.22.7269-7274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Higgins C. F., Pearce S. R., Gallagher M. P., Hoch J. A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991 Jan;5(1):173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Perego M., Spiegelman G. B., Hoch J. A. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988 Nov;2(6):689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Rao N. N., Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990 Jul;4(7):1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Roggiani M., Hahn J., Dubnau D. Suppression of early competence mutations in Bacillus subtilis by mec mutations. J Bacteriol. 1990 Jul;172(7):4056–4063. doi: 10.1128/jb.172.7.4056-4063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner D. Z., LeDeaux J. R., Ireton K., Grossman A. D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991 Feb;173(4):1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Formation of competent Bacillus subtilis cells. J Bacteriol. 1983 Feb;153(2):813–821. doi: 10.1128/jb.153.2.813-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Kunst F., Dedonder R. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976 Nov 17;148(3):281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kawata M. Cloning and characterization of Bacillus subtilis iep, which has positive and negative effects on production of extracellular proteases. J Bacteriol. 1988 Aug;170(8):3593–3600. doi: 10.1128/jb.170.8.3593-3600.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrauch Y., Guillen N., Dubnau D. A. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989 Oct;171(10):5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrauch Y., Msadek T., Kunst F., Dubnau D. Sequence and properties of comQ, a new competence regulatory gene of Bacillus subtilis. J Bacteriol. 1991 Sep;173(18):5685–5693. doi: 10.1128/jb.173.18.5685-5693.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrauch Y., Penchev R., Dubnau E., Smith I., Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990 May;4(5):860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Zuber P., Perkins J. B., Sandman K., Igo M., Losick R. New ways to study developmental genes in spore-forming bacteria. Science. 1985 Apr 19;228(4697):285–291. doi: 10.1126/science.228.4697.285. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D., Withoff S., Boels H., Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990 Dec;224(3):396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]


