Abstract
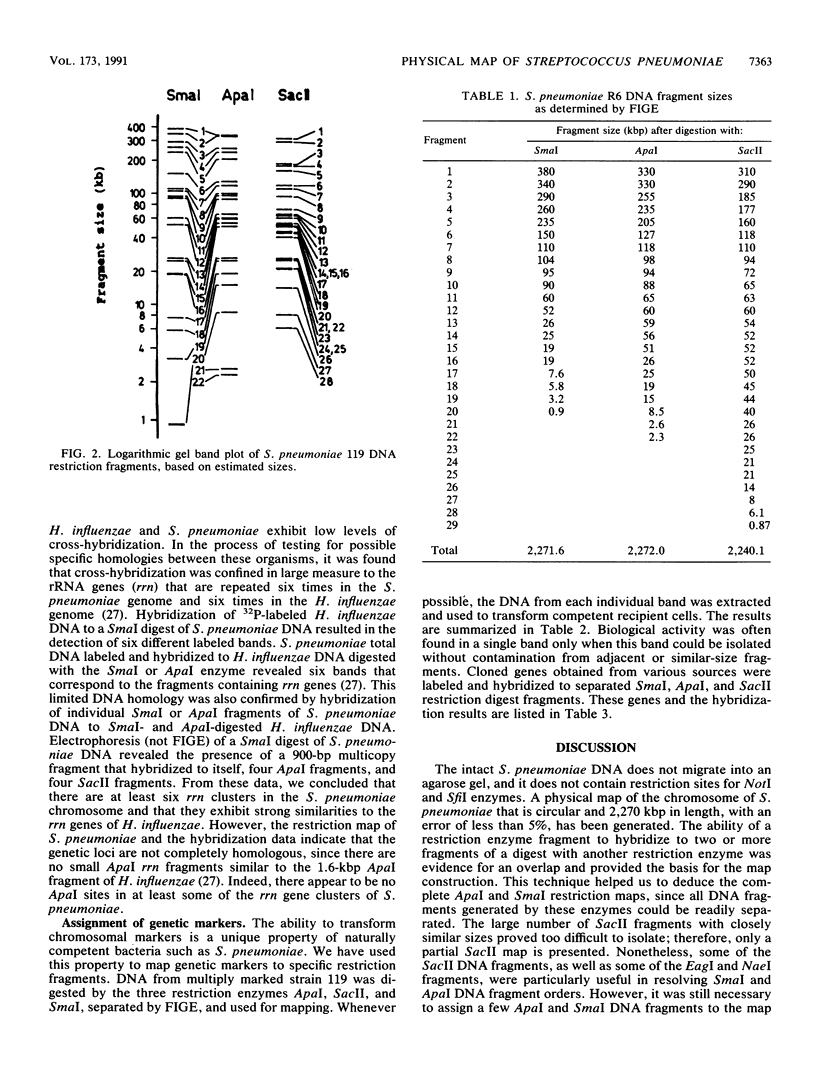
A physical map of the Streptococcus (Diplococcus) pneumoniae chromosome, which is circular and 2,270 kbp in circumference, has been constructed. The restriction enzymes ApaI, SmaI, and SacII were used to digest intact chromosomes, and the fragments were resolved by field inversion gel electrophoresis (FIGE). The digests produced 22, 20, and 29 fragments, respectively. The order of the fragments was deduced from Southern blot hybridization of isolated labeled fragments to separated fragments of the various restriction digests. Genetic markers were correlated with the physical map by transformation of recipient cells with FIGE-isolated DNA fragments derived from genetically marked S. pneumoniae strains. In addition, markers were mapped by the hybridization of cloned genes to FIGE-separated restriction fragments. Six rRNA gene (rrn) clusters were mapped by hybridization to rrn-containing fragments of Haemophilus influenzae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bućan M., Yang-Feng T., Colberg-Poley A. M., Wolgemuth D. J., Guenet J. L., Francke U., Lehrach H. Genetic and cytogenetic localisation of the homeo box containing genes on mouse chromosome 6 and human chromosome 7. EMBO J. 1986 Nov;5(11):2899–2905. doi: 10.1002/j.1460-2075.1986.tb04585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. S., Morrison D. A. Competence for genetic transformation in Streptococcus pneumoniae: molecular cloning of com, a competence control locus. J Bacteriol. 1987 May;169(5):2005–2011. doi: 10.1128/jb.169.5.2005-2011.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J. P., Lacks S. A. Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev. 1986 Jun;50(2):133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J. P., Roger M., Sicard A. M. Excision and repair of mismatched base pairs in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1980 Apr;178(1):191–201. doi: 10.1007/BF00267229. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Brannigan J. A., George R. C., Hansman D., Liñares J., Tomasz A., Smith J. M., Spratt B. G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 1989 Sep 25;17(18):7518–7518. doi: 10.1093/nar/17.18.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Gerardot C. J. Use of pulsed-field-gradient gel electrophoresis to construct a physical map of the Caulobacter crescentus genome. Gene. 1988 Sep 7;68(2):323–333. doi: 10.1016/0378-1119(88)90035-2. [DOI] [PubMed] [Google Scholar]
- García P., García J. L., García E., López R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43(3):265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- Gasc A. M., Sicard A. M., Claverys J. P. Repair of single- and multiple-substitution mismatches during recombination in Streptococcus pneumoniae. Genetics. 1989 Jan;121(1):29–36. doi: 10.1093/genetics/121.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc A. M., Sicard N., Claverys J. P., Sicard A. M. Lack of SOS repair in Streptococcus pneumoniae. Mutat Res. 1980 Apr;70(2):157–165. doi: 10.1016/0027-5107(80)90155-4. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauc L., Goodgal S. H. The size and a physical map of the chromosome of Haemophilus parainfluenzae. Gene. 1989 Nov 30;83(2):377–380. doi: 10.1016/0378-1119(89)90125-x. [DOI] [PubMed] [Google Scholar]
- Kauc L., Mitchell M., Goodgal S. H. Size and physical map of the chromosome of Haemophilus influenzae. J Bacteriol. 1989 May;171(5):2474–2479. doi: 10.1128/jb.171.5.2474-2479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laible G., Hakenbeck R. Penicillin-binding proteins in beta-lactam-resistant laboratory mutants of Streptococcus pneumoniae. Mol Microbiol. 1987 Nov;1(3):355–363. doi: 10.1111/j.1365-2958.1987.tb01942.x. [DOI] [PubMed] [Google Scholar]
- Laible G., Hakenbeck R., Sicard M. A., Joris B., Ghuysen J. M. Nucleotide sequences of the pbpX genes encoding the penicillin-binding proteins 2x from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol Microbiol. 1989 Oct;3(10):1337–1348. doi: 10.1111/j.1365-2958.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Le Bourgeois P., Mata M., Ritzenthaler P. Genome comparison of Lactococcus strains by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1989 May;50(1-2):65–69. doi: 10.1016/0378-1097(89)90460-6. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre J. C., Claverys J. P., Sicard A. M. Donor deoxyribonucleic acid length and marker effect in pneumococcal transformation. J Bacteriol. 1979 Apr;138(1):80–86. doi: 10.1128/jb.138.1.80-86.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Lacks S. A. Physical structure and genetic expression of the sulfonamide-resistance plasmid pLS80 and its derivatives in Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1984;195(3):403–410. doi: 10.1007/BF00341440. [DOI] [PubMed] [Google Scholar]
- Martin B., Prats H., Claverys J. P. Cloning of the hexA mismatch-repair gene of Streptococcus pneumoniae and identification of the product. Gene. 1985;34(2-3):293–303. doi: 10.1016/0378-1119(85)90138-6. [DOI] [PubMed] [Google Scholar]
- Martinez S., Lopez P., Espinosa M., Lacks S. A. Cloning of a gene encoding a DNA polymerase-exonuclease of Streptococcus pneumoniae. Gene. 1986;44(1):79–88. doi: 10.1016/0378-1119(86)90045-4. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Rives I., Claverys J. P. Nucleotide sequence of the Streptococcus pneumoniae ung gene encoding uracil-DNA glycosylase. Nucleic Acids Res. 1990 Nov 25;18(22):6693–6693. doi: 10.1093/nar/18.22.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats H., Martin B., Claverys J. P. The hexB mismatch repair gene of Streptococcus pneumoniae: characterisation, cloning and identification of the product. Mol Gen Genet. 1985;200(3):482–489. doi: 10.1007/BF00425735. [DOI] [PubMed] [Google Scholar]
- Priebe S. D., Hadi S. M., Greenberg B., Lacks S. A. Nucleotide sequence of the hexA gene for DNA mismatch repair in Streptococcus pneumoniae and homology of hexA to mutS of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 Jan;170(1):190–196. doi: 10.1128/jb.170.1.190-196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudhomme M., Martin B., Mejean V., Claverys J. P. Nucleotide sequence of the Streptococcus pneumoniae hexB mismatch repair gene: homology of HexB to MutL of Salmonella typhimurium and to PMS1 of Saccharomyces cerevisiae. J Bacteriol. 1989 Oct;171(10):5332–5338. doi: 10.1128/jb.171.10.5332-5338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee D. K., Morrison D. A. Genetic transformation in Streptococcus pneumoniae: molecular cloning and characterization of recP, a gene required for genetic recombination. J Bacteriol. 1988 Feb;170(2):630–637. doi: 10.1128/jb.170.2.630-637.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICARD A. M. A NEW SYNTHETIC MEDIUM FOR DIPLOCOCCUS PNEUMONIAE, AND ITS USE FOR THE STUDY OF RECIPROCAL TRANSFORMATIONS AT THE AMIA LOCUS. Genetics. 1964 Jul;50:31–44. doi: 10.1093/genetics/50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C., Dobrinski B., Hakenbeck R. Unusual septum formation in Streptococcus pneumoniae mutants with an alteration in the D,D-carboxypeptidase penicillin-binding protein 3. J Bacteriol. 1990 Nov;172(11):6499–6505. doi: 10.1128/jb.172.11.6499-6505.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A. M. Gene conversion in Streptococcus pneumoniae. Microbiologia. 1987 Feb;3(1):5–12. [PubMed] [Google Scholar]
- Sicard N., Estevenon A. M. Excision-repair capacity in Streptococcus pneumoniae: cloning and expression of a uvr-like gene. Mutat Res. 1990 May;235(3):195–201. doi: 10.1016/0921-8777(90)90074-f. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby G., Sicard M. A. Integration efficiency in DNA-induced transformation of Pneumococcus. II. Genetic studies of mutant integrating all the markers with a high efficiency. Genetics. 1973 Sep;75(1):35–48. doi: 10.1093/genetics/75.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Fox M. S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar M. N., Priebe S. D., Guild W. R. Structure of a conjugative element in Streptococcus pneumoniae. J Bacteriol. 1986 Jun;166(3):978–984. doi: 10.1128/jb.166.3.978-984.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




