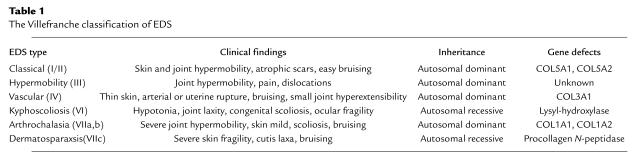
The Ehlers-Danlos syndrome (EDS) is a clinically and genetically heterogeneous connective tissue disorder affecting as many as 1 in 5,000 individuals (1). EDS is characterized in its most common form by hyperextensibility of the skin, hypermobility of joints often resulting in dislocations, and tissue fragility exemplified by easy bruising, atrophic scars following superficial injury, and premature rupture of membranes during pregnancy. The recognition of frequent ultrastructural abnormalities of collagen fibrils in EDS patients led to the concept that EDS is a disorder of fibrillar collagen metabolism (2). Following the identification of specific mutations in the genes encoding collagen types I, III, and V, as well as several collagen processing enzymes, the EDS classification scheme was collapsed into six distinct clinical syndromes (3), emphasizing the molecular basis of each form (Table 1).
Table 1.
The Villefranche classification of EDS
Heterogeneity between the several clinical syndromes both complicates the diagnosis of EDS and makes accurate diagnosis imperative. Ultimately, one would like to be able to establish a molecular diagnosis for each EDS patient. This is a laudable goal because it may allow improved genetic counseling through correlation of mutant genotypes with specific outcomes or complications. However, as outlined below, the molecular defects described to date are not sufficient to explain disease in many EDS patients, including those with the most common classical and hypermobility types. As a result, the search for EDS genes recently has expanded beyond the collagens and collagen-modifying genes. An understanding of the complete complement of genes and proteins involved in EDS and the precise mechanisms by which they cause disease may teach us much about normal collagenous matrix deposition and remodeling. These processes are of critical importance during development, wound healing, and aging.
Collagen metabolism and molecular mechanisms of EDS
The concept that EDS is a disorder of fibrillar collagen metabolism is well supported by identification of specific defects in the collagen biosynthetic pathway that produce clinically distinct forms of EDS. These are briefly reviewed below and in Figure 1. For more complete reviews of collagen biochemistry and clinical aspects of EDS, the reader is directed to recent reviews (1, 2).
Figure 1.
The biosynthetic pathway for the fibrillar collagens expressed in skin, identifying steps that are affected in different forms of EDS. (I) Collagen gene transcription is highly regulated, but haploinsufficiency for COL5A1 is uncompensated and leads to a reduction in COL5A1 mRNA and α1(V) procollagen chains. This accounts for 30–50% of classical EDS cases. (II) Many proline and lysine residues in the translated procollagen chains are hydroxylated by lysyl- and proline hydroxylases. Hydroxylation is essential for subsequent crosslinking and lysyl-hydroxylase deficiency causes the kyphoscoliosis form of EDS. (III) Procollagen α-chains are assembled into trimers within the rough endoplasmic reticulum (RER). Mutations in COL3A1 that interrupt the triple helical structure prevent normal processing and secretion of collagen III, causing the vascular form of EDS. (IV) In the ECM, the NH2- and COOH-terminal propeptides are cleaved by specific peptidases. Dominant mutations in COL1A1 and COL1A2 can prevent cleavage and cause arthrochalasia, while recessive loss of the N-procollagen peptidase cause dermatosparaxis. (V) Collagen molecules self-assemble into heterotypic fibrils. Dominant-negative mutations in COL5A1 and COL5A2 alter fibril assembly and cause some cases of classical EDS. (VI) Collagen fibrils are deposited in tissue-specific arrangements in close association with many fibril-associated proteins and proteoglycans. Because new fibrils are laid down in close association with the fibroblast cell membrane, interactions between the fibril and the cell are important and may involve direct interaction with collagens and/or matricellular proteins, including tenascin-X (TNX).
There are three fundamental mechanisms of disease known to produce EDS: deficiency of collagen-processing enzymes, dominant-negative effects of mutant collagen α-chains, and haploinsufficiency. The two known examples of deficient enzyme activity leading to EDS are lysyl-hydroxylase deficiency and procollagen peptidase deficiency. In the first case, the inability to hydroxylate lysine residues precludes normal intermolecular cross-linking of collagen trimers, and in the second instance, absence of procollagen peptidase prevents normal proteolytic cleavage of the NH2-terminus of procollagen chains. In both circumstances the morphology and strength of the collagen fibril is compromised (Figure 2b), explaining the severe and early clinical findings. Because half-normal enzyme activity is sufficient for normal collagen processing, both of these conditions are recessive.
Figure 2.
The collagen fibril and EDS. (a) Normal collagen fibrils are of uniform size and spacing. Fibrils from a patient with dermatosparaxis (b) show dramatic alterations in fibril morphology with severe effects on tensile strength of connective tissues. Most fibrils from a patient with classical EDS (c) are normal in appearance. Composite fibrils (arrows) are typically rare. Fibrils from a TNX-deficient patient (d) are uniform in size and no composite fibrils are seen. When compared with normal skin (e), TNX-null fibrils are less densely packed and not as well aligned to neighboring fibrils. In normal skin (e) and cornea (f), fibrils are deposited in tissue-specific patterns. In skin, bundles of fibrils are oriented in different directions to resist forces in multiple axes. In cornea, orthogonal arrays allow maximal transparency.
Dominant-negative mutations in collagen I, III, and V cause several different forms of EDS (Figure 1). The most clinically significant of these are COL3A1 mutations causing the potentially lethal vascular form of EDS. The reported COL3A1 mutations are either exon-skipping mutations or missense mutations that disrupt the collagen triple helix (4). Because collagen III consists of homotrimers and the normal and mutant pro α1(III) chains appear to be produced in equal abundance, nearly 90% of all α1(III) trimers will contain one or more mutant α-chains. Trimers containing mutant chains are not secreted and are either degraded or accumulate to high levels in intracellular compartments. Despite the fact that collagen III is a minor component of dermal collagen fibrils, the severe reduction in collagen III leads to small or variably sized collagen fibrils and thinning of the dermis. The clinical phenotype suggests an important role for collagen III in either the synthesis or deposition of heterotypic fibrils containing largely collagen I, but remarkably, the precise mechanism of disease is still unknown. Because collagen III is an important component of the arterial and bowel walls, these tissues are also frequently affected.
Exon-skipping and missense mutations in the COL5A1 and COL5A2 genes have also been reported to cause classical EDS (5, 6), presumably through dominant-negative effects. However, two recent reports demonstrated that COL5A1 haploinsufficiency appears to be more common (7, 8). These studies showed that one-third of a subset of classical EDS patients that could be evaluated (because they are heterozygous for polymorphisms of COL5A1) did not express the mutant allele. Mutant COL5A1 transcripts are presumably degraded through non-sense–mediated RNA decay. Truncating mutations were identified subsequently in most haploinsufficient patients. The dramatic clinical effect of COL5A1 haploinsufficiency is surprising, given that collagen V is a quite minor constituent of collagen in skin, tendon, and ligament, but the clinical phenotype clearly supports an important function for collagen V in the maintenance of biomechanical integrity of collagenous matrix.
One clearly established function of collagen V is limitation of fibril diameter. When mixtures of denatured collagen I and collagen V are renatured in vitro, fibrils of smaller size are produced with increasing collagen V content (9). Indeed, the ultrastructural hallmark of classical EDS is a 25% increase in fibril diameter and rare composite fibrils, often called “collagen cauliflowers” (Figure 2c). While the effect of collagen V on fibril diameter is intrinsic to its structure and does not require enzymatic activity or the presence of noncollagen proteins, the mechanism by which collagen V regulates fibril size is uncertain. The α1(V) chain has a larger than usual NH2-terminal nonhelical domain, and pepsin cleavage of this domain eliminates the effect of collagen V on fibril diameter (10). Further, immunohistochemical studies demonstrate that the triple helical region of collagen V is buried within the fibril, and the amino terminus of α1(V) protrudes from the fibril surface. Because the amino terminus of α1(V) contains numerous sulfated tyrosine residues, this domain carries a significant negative charge. It is postulated that this domain’s bulk or negative charge density limits growth of the nascent fibril once a critical number of collagen V trimers are incorporated.
It is not clear how the relatively minor abnormalities in fibril structure, often affecting less than 5% of fibrils, lead to rather profound alterations of connective tissue function. It is quite possible that the alterations in fibril structure are but a marker of the disorder and that the more important effects of COL5A1 haploinsufficiency involve additional unknown functions of collagen V. One such possible function is Vregulation of the higher order, tissue-specific arrangement of collagen fibrils (Figure 2, e and f). Biomechanical studies of EDS skin suggest that hyperextensibility is more likely to reflect fibril disarray than extensibility of collagen fibrils themselves (11). Remarkably, while we know a great deal about the biochemistry of collagen synthesis, we know virtually nothing about how fibrils are deposited into tissue-specific forms. Collagen V might participate in this process through interactions with other fibril-associated proteins or proteoglycans (e.g., decorin; ref. 12), interactions with the cell surface (e.g., integrins or the discoidin domain receptor tyrosine kinases; ref. 13), or both.
A final issue with respect to collagen V mutations and classical EDS is the question of what fraction of patients actually carry mutations in the COL5A1 or COL5A2 genes. Initial enthusiasm that such mutations accounted for all or most classical EDS has been restrained by subsequent findings, which suggest that 30–50% of classical cases are caused by COL5A1 mutations and a substantially smaller number by COL5A2 mutations. Further, the COL5A1 and COL5A2 genes have been excluded by linkage analysis of some families (14, 15). Recent cloning of the COL5A3 gene will make possible mutational analysis of this gene (16), but it is unlikely that all remaining cases of classical EDS will be accounted for by COL5A3 mutations. Hence, it seems likely to us that other causes of classical EDS exist and involve genes encoding proteins other than the fibrillar collagens or collagen-modifying enzymes.
Tenascin-X and EDS
The tenascins are a family of large matricellular proteins of unknown function (17). The three family members described in mammals are tenascin-C (TNC, formerly cytotactin, or tenascin), tenascin-R (TNR, restrictin) and tenascin-X (TNX). All family members share a modular structure consisting of an NH2-terminal sequence responsible for oligomerization of TNC, TNR, and possibly TNX; a variable number of cysteine-rich EGF-like repeats; repeats similar to the type III repeats of fibronectin; and a COOH-terminal fibrinogen-like domain. The biomedical literature includes more than 1,000 references concerning these proteins that suggest many different functions. Given the expected importance of these proteins, especially during development, the very mild phenotypes of TNC- and TNR-null mice were unexpected (18–20). Functional redundancy between family members has been postulated, and interest has been focused on TNX.
TNX was cloned serendipitously because of its 3′ overlap with the human CYP21 gene encoding steroid 21-hydroxylase (21, 22), and subsequent intensive study of this locus revealed an extraordinary level of complexity (Figure 3). Analysis of the pattern of expression of TNX in rodents and pig demonstrated that it is highly expressed in connective tissue of muscle during development, as well as around tendons, ligaments, and in skin (23, 24). Comparison of TNX and TNC expression patterns suggested partially overlapping expression or adjacent expression in many tissues including skin.
Figure 3.
A novel deletion in the C4/CYP21/TNX/CREB-rp locus is associated with EDS. The C4 genes, the CYP21 genes and part of TNX are duplicated on chromosome 6. Dashed lines indicate the limits of the duplication event. The normal locus is shown in the top panel. Arrows indicate direction of transcription. TNX is overlapped at its 3′ end by the CYP21B (21B) gene encoding steroid 21-hydroxylase and, at its 5′ end, by CREB-related protein (CREB-rp). XA is a partial duplicate of TNX that is a pseudogene transcribed but not translated in the human adrenal. XA contains a 121 bp deletion (Δ) that truncates the open reading frame corresponding to TNX. CYP21A (21A) is a pseudogene. One-quarter of CYP21B-deficient alleles carry a 30 kb deletion extending from CYP21A to CYP21B (middle panel). This creates a nonfunctional CYP21AB fusion gene and deletes XA, but does not alter TNX. We described a similar deletion (lower panel) extending from XA to TNX that completely deletes CYP21B and creates a TNX/XA fusion gene. This deletion is associated with a new contiguous gene syndrome consisting of congenital adrenal hyperplasia, due to CYP21B deficiency, and EDS, due to TNX deficiency.
Because biochemical studies had failed to reveal unequivocal functions for TNC and TNR, our laboratory undertook a genetic approach to identify disease caused by TNX in humans. We relied on the fact that CYP21 deficiency causes the well-studied human disease congenital adrenal hyperplasia (CAH) that results from the inability to synthesize cortisol, and the consequent overproduction of androgenic steroids. The molecular genetics of CAH is of special interest because 75% of mutant alleles arise from gene conversion events in which CYP21 sequences are replaced by corresponding sequences of the CYP21A pseudogene. The remaining 25% of CYP21 mutant alleles are deletions that arise from homologous recombination between the two CYP21 genes that lie 30 kb apart (Figure 3, middle panel). We reasoned that similar recombination events might occur between TNX and its highly homologous pseudogene XA (Figure 3, lower panel). The resulting TNX/XA fusion gene cannot encode full-length TNX because a 121 bp deletion truncates the XA open reading frame (25). This deletion completely removes CYP21, so alleles carrying this mutation might be found in CAH patients. Therefore we sought patients with both CAH and a connective tissue disorder that might be due to abnormalities in TNX. We identified one such patient (26) and confirmed that he carried the predicted deletion on his paternal allele. We also showed that TNX mRNA and protein were completely lacking in skin and cultured dermal fibroblasts from this individual. The absence of TNX protein suggested recessive inheritance, a concept bolstered by the fact that the index patient’s clinically normal parents and two siblings each share an extended TNX/CYP21 haplotype with the proband.
Since publication of our initial report, we have identified two additional patients with CAH and EDS, and all three patients have similar EDS phenotypes (our unpublished results). The essential features are typical skin and joint hyperextensibility and easy bruising. Features that may distinguish these patients from other classical EDS patients are the absence of atrophic scars, absence of collagen cauliflowers on ultrastructural examination of skin, and the recessive pattern of inheritance. However, because of the coexistence of CAH with EDS in these patients, it is not yet clear that isolated TNX deficiency causes EDS in humans. Large scale screening of an unselected EDS cohort is underway. Similarly, because mutational analysis is incomplete, the recessive nature of TNX deficiency is likely, but not proven.
The association of TNX deficiency with EDS is exciting because it simultaneously establishes the first clinically relevant function for any of the tenascins and immediately broadens the search for EDS candidate genes beyond the collagens and their modifying enzymes. However, many important questions remain, including clarification of the mechanisms though which TNX deficiency causes disease. Several possibilities exist. First, TNX may play a primary structural role in collagenous matrix. TNX appears to be fibril-associated and is localized in the spaces between fibrils by immunology electron microscopy of bovine and human skin. A structural model of the fibronectin type III repeats of TNX presented by Erickson suggests that these elements may undergo reversible unfolding and refolding (27). TNX has more than 30 such repeats, so it has the potential to be quite flexible (22). In addition, TNX has been predicted to exist in a trimeric form, so one possible model is that TNX oligomers bind to a component of collagen fibrils and provide a flexible link between them. Loss of TNX could reasonably be expected to lead to increased distensibility and reduced tensile strength of collagenous matrix.
A second possibility is that TNX interacts with cells to influence synthesis of collagens or other matrix elements. Like other matricellular proteins, it seems likely that TNX interacts with cells through specific receptors. Purified bovine TNX has been shown to bind to a β1 integrin, although the relevant α-chain has not yet been identified (28). Human and bovine TNX contain an RGD sequence, which, in other ECM proteins, binds to integrins, but mouse TNX lacks this tripeptide, so it is not clear whether this sequence has functional importance. Whether TNX triggers intracellular signaling events or alters synthesis of specific matrix components is unknown, but the index patient’s fibroblasts appear to synthesize collagen I and collagen V normally (26).
A third possibility is that TNX alters the higher organization of collagen fibril deposition. Ultrastructural analysis of collagen fibrils from a TNX-null patient does show mild derangement of fibril organization (Figure 2d). This effect might be mediated through TNX interactions with the fibril, with the fibroblast, or more likely with both. As noted above, there is very little known about how the tissue-specific architecture of ECM is established, and fibril-associated matricellular proteins must surely be the best candidates for such a role. Given the absence of demonstrated abnormalities in fibril morphology or collagen synthesis, this mechanism may be the most plausible.
Utility of the mouse for studies of matrix function and EDS
Our relatively poor understanding of the molecular pathology in EDS, even when the mutations are known, reflects the fact that patient samples are necessarily limited to small amounts of skin obtained at biopsy and cultured cells. Animal models of most forms are not available for study, but the genetic manipulability of the mouse has begun to rectify this situation. The introduction of specific mutations through gene targeting should provide animal models for testing specific hypotheses regarding EDS pathogenesis. This approach will also allow for proof of causation when a new gene is found to be associated with EDS and aid in the identification of new candidate genes for human EDS.
An example of how our understanding of molecular mechanisms can be expanded by mouse models comes from the COL3A1 knockout mouse (29). This mutation in mice nicely recapitulates the human vascular EDS phenotype, including thin and fragile skin, aortic aneurysm and rupture, and abnormal collagen fibrillogenesis in skin, aorta, and bowel. The homozygous null mutation in mice is somewhat more severe than the dominant-negative effect in humans because the loss of collagen III protein is complete in the mouse. Because the mouse tissues were readily available for study, the investigators were able to evaluate collagen content and fibril morphology in a number of tissues including the aorta. The surprising result was that fibril number was reduced by two-thirds in the aortic adventia of knockout animals, suggesting a previously unrecognized role for collagen III in regulating the number of fibrils deposited in addition to their diameter.
Mouse models can provide information to support causation of EDS when an association exists in only a small number of patients. This has been the case for TNX, where the association was found in a single patient with a complex phenotype due to his contiguous gene syndrome and where the genetics was not clear. Recently we inactivated TNX in mice. TNX-deficient mice are fertile and viable, and they recapitulate the skin manifestations of classical EDS. TNX-null mice exhibit progressive skin hyperextensibility and reduced tensile strength with minimal alteration in fibril morphology (our unpublished observations). Heterozygous mice appear to be normal, as predicted from the recessive pattern in the human index case. These observations strongly suggest that isolated TNX deficiency can cause EDS and justify the rather substantial task of screening a classical EDS population for null mutations in this large and highly repetitive gene.
It should be noted that the nature of the mutation introduced into the mouse may have important consequences for the observed phenotype. This is demonstrated by the introduction of a mutation into exon 6 of the COL5A2 gene that prevents normal cleavage of the NH2-terminal propeptide (30). While a dominant-negative effect might have been anticipated, heterozygous mice are normal, while homozygous mice have severe defects of cornea, mildly impaired growth, skin fragility, and mild skin extensibility — a substantially different phenotype from classical EDS due to COL5A1 haploinsufficiency. It remains to be seen whether a true COL5A1 null will recapitulate classical EDS in mice.
Finally, the mouse has repeatedly yielded evidence of unexpected nonredundant functions of individual genes, and as such, gene targeting in the mouse has become an increasingly important means of recognizing new candidate genes for many human diseases, including EDS. Several recent examples serve to illustrate this point.
The first examples are gene knockouts of three small leucine-rich proteoglycans (SLRPs), decorin, fibromodulin, and lumican (31–33). The phenotypes of all three knockouts include derangements in collagen fibril morphology. Curiously, the fibril abnormalities are quite different in the three animal models. Fibromodulin-null fibrils are smaller than wild-type fibrils and lumican-null fibrils are somewhat larger than normal, while decorin-null fibrils are of similar size but have greater size variation than normal fibrils. In the case of decorin and lumican, the defect in fibrillogenesis is accompanied by clinically apparent skin hyperextensibility and a striking reduction in skin tensile strength. Further support for these genes as candidates in EDS comes from the observation that all are fibril-associated and the observation that SLRPs at the surface of the fibril appear to limit fibril growth and lateral fusion of fibrils (34). Clearly testing of patients for mutations in these genes is warranted.
Two important recent examples are the knockouts of the matricellular proteins thrombospondin-2 (TSP2) (35) and SPARC (secreted protein acidic and rich in cysteine) (36). The biology of both proteins have been reviewed in detail in other Perspectives in this series (Bornstein, this series, ref. 37; Bradshaw and Sage, this series, ref. 38). Both mice have distorted collagen fibrillogenesis and hyperextensible skin with reduced tensile strength. In addition, the TSP2-null mouse has joint laxity, as is dramatically demonstrated by the ability to tie their tails into knots. SPARC is clearly fibril-associated and, while TSP2 is not known to be fibril-associated, TSP1 can bind to purified collagens. Because these proteins interact with both the cell surface and with ECM, understanding the mechanism of skin and joint abnormalities in these mice may provide important new insights into how collagenous matrices are deposited and remodeled. Clearly these genes also deserve consideration as EDS candidates.
What the future holds
It seems highly likely that genes other than the fibrillar collagens and their known modifying enzymes participate in the complex process of collagen fibrillogenesis and establishment of matrix architecture. Given that much of EDS is still unexplained, it seems equally likely that other noncollagen genes will be discovered to cause disease in some fraction of EDS patients. Defects in some genes, like COL5A1 and the genes encoding the SLRPs, will produce alterations in fibril morphology, while others may produce the syndrome with minimal or no alteration in the fibril, as is the case for TNX defects.
Some new candidate genes will probably be identified serendipitously through gene targeting in the mouse, but a more directed strategy might begin with examination of logical candidates, such as the fibril-associated proteins. Many of these are matricellular proteins with both matrix and cellular interactions, and the interacting matrix proteins and cellular receptors can be identified and included in the candidate list. It is hoped that much of the pathway from collagen secretion to matrix superstructure can be outlined in this way.
A second potentially useful approach is expression profiling of materials from patients. Using cDNA microarrays (39), the relative expression of thousands of genes can be examined in cell lines derived from patients with known or unknown mutations and a set of misregulated genes can be identified. EDS is ideally suited for this kind of analysis because one of the affected tissues (skin) is readily sampled without undue risk or inconvenience for patients. We expect that some fraction of the misregulated genes will be new components of the collagen deposition/ remodeling pathway. This strategy has the additional advantage that an expression fingerprint may be identified for cell lines with mutations in a specific gene, perhaps allowing rapid diagnosis with less labor than is presently possible.
Clearly there is much work to be done before complete genetic analysis of EDS can become routine. However, the tools now exist to achieve this and progress is being made rapidly. In the process of fully defining the genetic basis of the EDS clinical syndromes, much will be learned about collagen matrix deposition and remodeling with important implications for the establishment of tissue architecture during development and other clinical problems such as wound healing.
Acknowledgments
This work was supported by grants from the NIH (HL 02695) and the March of Dimes Birth Defects Foundation.
References
- 1.Steinmann, B., Royce, P., and Superti-Furga, A. 1993. The Ehlers-Danlos Syndrome. In Connective tissue and its heritable disorders. P. Royce and B. Steinmann, editors. Wiley-Liss. New York, New York, USA. 351–407.
- 2.Byers, P. 1995. Disorders of collagen biosynthesis and structure. In The metabolic basis of inherited disease. C. Scriver, A. Beaudet, W. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 4029–4075.
- 3.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Pepin M, Schwarze U, Superti-Furga A, Byers P. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls AC, et al. An exon skipping mutation of a type V collagen gene (COL5A1) in Ehlers-Danlos syndrome. J Med Genet. 1996;33:940–946. doi: 10.1136/jmg.33.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalickova K, Susic M, Willing MC, Wenstrup RJ, Cole WG. Mutations of the alpha2(V) chain of type V collagen impair matrix assembly and produce ehlers-danlos syndrome type I. Hum Mol Genet. 1998;7:249–255. doi: 10.1093/hmg/7.2.249. [DOI] [PubMed] [Google Scholar]
- 7.Wenstrup R, et al. COL5A1 haploinsufficiency is a common molecular mechanism underlying the classical form of EDS. Am J Hum Genet. 2000;66:1766–1776. doi: 10.1086/302930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarze U, Atkinson M, Hoffman GG, Greenspan DS, Byers PH. Null alleles of the COL5A1 gene of type V collagen are a cause of the classical forms of Ehlers-Danlos syndrome (types I and II) Am J Hum Genet. 2000;66:1757–1765. doi: 10.1086/302933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birk D, Fitch J, Babiarz J, Doane K, Linsenmayer T. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 10.Linsenmayer T, et al. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grahame R, Beighton P. Physical properties of the skin in the Ehlers-Danlos syndrome. Ann Rheum Dis. 1969;28:246–251. doi: 10.1136/ard.28.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischmajer R, et al. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991;106:82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- 13.Vogel W, Gish G, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 14.Wenstrup RJ, Langland GT, Willing MC, D’Souza VN, Cole WG. A splice-junction mutation in the region of COL5A1 that codes for the carboxyl propeptide of pro alpha 1(V) chains results in the gravis form of the Ehlers-Danlos syndrome (type I) Hum Mol Genet. 1996;5:1733–1736. doi: 10.1093/hmg/5.11.1733. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan DS, et al. COL5A1: fine genetic mapping and exclusion as candidate gene in families with nail-patella syndrome, tuberous sclerosis 1, hereditary hemorrhagic telangiectasia, and Ehlers-Danlos Syndrome type II. Genomics. 1995;25:737–739. doi: 10.1016/0888-7543(95)80021-d. [DOI] [PubMed] [Google Scholar]
- 16.Imamura Y, Scott I, Greenspan D. The pro-alpha3(V) collagen chain: complete primary structure, expression domains in adult and developing tissues, and comparison to the structures and expression domains of the other types V and XI procollagen chains. J Biol Chem. 2000;275:8749–8759. doi: 10.1074/jbc.275.12.8749. [DOI] [PubMed] [Google Scholar]
- 17.Jones F, Jones P. The tenascin family of ECM glycoproteins: structure, function and regulation during embryonic development and tissue remodeling. Develop Dynam. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg E, et al. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci USA. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber P, et al. Mice deficient in tenascin-R display alteration of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci. 1999;19:4245–4262. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel Y, Bristow J, Gitelman SE, Miller WL. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci USA. 1989;86:6582–6586. doi: 10.1073/pnas.86.17.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993;122:265–278. doi: 10.1083/jcb.122.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Saga Y, Ikemura T, Sakakura T, Chiquet-Ehrismann R. The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J Cell Biol. 1994;125:483–493. doi: 10.1083/jcb.125.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geffrotin C, Garrido JJ, Tremet L, Vaiman M. Distinct tissue distribution in pigs of tenascin-X and tenascin-C transcripts. Eur J Biochem. 1995;231:83–92. doi: 10.1111/j.1432-1033.1995.tb20673.x. [DOI] [PubMed] [Google Scholar]
- 25.Gitelman SE, Bristow J, Miller WL. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol. 1992;12:2124–2134. doi: 10.1128/mcb.12.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burch GH, et al. Tenascin-X deficiency is associated with Ehlers-Danlos Syndrome. Nat Genet. 1997;17:104–108. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- 27.Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA. 1994;91:10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elefteriou F, Exposito JY, Garrone R, Lethias C. Cell adhesion to tenascin-X mapping of cell adhesion sites and identification of integrin receptors. Eur J Biochem. 1999;263:840–848. doi: 10.1046/j.1432-1327.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrikopoulos K, Liu X, Keene D, Jaenisch R, Ramirez F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995;9:31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- 31.Danielson K, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson L, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarti S, et al. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel K, Trotter J. The effects of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 35.Kyriakides TR, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradshaw A, Francki A, Motamed K, Howe C, Sage E. Primary mesenchymal cells isolated from SPARC-null mice exhibit altered morphology and rates of proliferation. Mol Biol Cell. 1999;10:1569–1579. doi: 10.1091/mbc.10.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisen MB, Brown PO. DNA arrays for analysis of gene expression. Methods Enzymol. 1999;303:179–205. doi: 10.1016/s0076-6879(99)03014-1. [DOI] [PubMed] [Google Scholar]