Abstract
Docosahexaenoic acid (DHA; 22:6 n-3) is an essential fatty acid required for the normal function of several tissues, especially the brain. Previous studies suggested that lysophosphatidylcholine (lysoPC) is a preferred carrier of DHA to the brain, although the pathways of the formation of DHA-containing lysophospholipids in plasma have not been delineated. We propose that endothelial lipase (EL), a phospholipase A1 that plays an important role in the metabolism of high density lipoproteins, may be responsible for the generation of DHA lysophospholipids in plasma. Here we studied the substrate specificity of EL using deuterium-labeled phospholipids with different polar head groups, as well as DHA-enriched natural phospholipids to test this hypothesis. Glycerol-stabilized phospholipids were treated with recombinant EL, and the products were analyzed by liquid chromatography/electrospray ionization mass spectrometry. EL showed the polar head group specificity in the order of phosphatidylethanolamine > phosphatidylcholine > phosphatidylserine > phosphatidic acid. Within the same phospholipid class, the enzyme showed preference for the species containing DHA at the sn-2 position, and was inactive in the hydrolysis of phospholipids containing an ether linkage. Since EL is known to be secreted by the cells of blood brain barrier, we suggest that it plays an important role in the delivery of DHA lysophospholipid carriers to the brain.
Keywords: docosahexaenoic acid, endothelial lipase, phosphatidylethanolamine, phosphatidylcholine, docosahexaenoyl lysophosphatidylcholine, docosahexaenoyl lysophosphatidylethanolamine
1. Introduction
The role of omega-3 polyunsaturated fatty acids, in particular docosahexaenoic acid (DHA; 22:6 n-3), in brain development and neurological function has been well established [1,2]. The deficiency of DHA in the brain markedly affects neurotransmission, membrane-bound enzyme and ion channel activities, leading to neurodegenerative diseases [3]. DHA in membrane phospholipids has profound effects on membrane raft composition and function, membrane receptor activities, and cellular signaling [4]. In addition, the physical properties of the membranes, such as fluidity, phase transition temperature and bilayer thickness are strongly affected by DHA [5]. Because the capacity of the brain to convert α-linolenic acid to DHA is limited, especially in immature animals, it has to be supplied in the diet or derived from other tissues [6,7]. Dietary n-3 fatty acid supplements including DHA, α-linolenic acid and eicosapentaenoic acid are widely recommended for various populations, especially the infants, elderly, and patients with neurological and mental disorders [8–10]. The amount of DHA that is actually taken up by brain after ingesting various types of supplements, however, is not clearly established. Unlike other tissues, the brain uptake needs to overcome the blood-brain barrier (BBB), and the transport mechanisms involved for DHA uptake through this barrier are poorly understood.
Studies with whole animals as well as with in vitro systems showed that DHA and other long chain polyunsaturated fatty acids (PUFA) are transported across the BBB more efficiently when they are esterified to lysophosphatidylcholine (lysoPC), compared to free PUFA [11–13]. Interestingly, this was specific for the PUFA, because the saturated fatty acids were transported to the same extent whether they were in the free form or esterified to lysoPC [14]. However, the pathways by which the DHA-lysoPC is formed in plasma compartment are not fully understood. The long chain PUFA are exclusively in the sn-2 position of plasma phospholipids, and therefore the action of phospholipases A2 (PLA2) would not generate DHA-lysophospholipids. We have previously shown that plasma lecithin-cholesterol acyltransferase (LCAT), which is quantitatively the most important enzyme in the hydrolysis of plasma PC, alters its positional specificity in the presence of sn-2 DHA-PC species, resulting in the formation of the sn-2 DHA-lysoPC [15,16]. In addition, Brossard et al. [17], and Lemaitre-Delaunay et al. [18] reported that DHA ingested as triglycerides and phospholipids appear as lysoPC in plasma at higher specific activities than PC, suggesting the presence of pathways that secrete DHA-lysoPC from liver or other tissues. Direct hepatic secretion of unsaturated lysoPC has also been reported in rats [19].
An additional source of sn-2 DHA-lysoPC may be through the action of endothelial lipase (EL), which is expressed in several tissues, and is anchored at the endothelial surface. Recent investigations show that it has more phospholipase activity than lipase activity, but unlike the phospholipases, it specifically hydrolyzes the sn-1 acyl groups from diacyl phosphoglycerides [20]. Thus the PUFA, which are mostly present in the sn-2 position of phospholipids, end up in lysophospholipids rather than in free fatty acids. This enzyme therefore could provide an alternate mechanism by which the sn-2 DHA-lysoPC can be generated in plasma. Furthermore, EL has been shown to be expressed and secreted by brain endothelial cells [21] and astrocytes, the major components of the BBB, and therefore could be involved in the generation of DHA-containing lysophospholipids at the BBB.
Although EL has been shown to be relatively more specific for phospholipids than triglycerides, studies on its phospholipid specificity are limited. Duong et al. [22], employing reconstituted HDL composed of single species of PC, reported the preferential hydrolysis of PC containing DHA. Gauster et al. [20] reported that in addition to exhibiting PLA1 activity, EL hydrolyzed some 2-acyl lysoPC generated during the reaction, thus releasing both saturated and unsaturated fatty acids. They also showed that the enzyme did not hydrolyze fatty acids from sn-1 ether PC, confirming the positional specificity of the enzyme for sn-1 acyl ester. However, the specificity of the enzyme for the polar head group of phospholipids was not analyzed. Furthermore, there is no information on the relative specificity of the enzyme in the natural mixtures of PC species. In this study we first analyzed the head group specificity of the enzyme using deuterated phospholipids of identical fatty acid composition. In addition, we studied the fatty acid specificity of the enzyme using phospholipids from a natural source that is rich in DHA-lipids, namely the shark liver lipids. The results presented here show that EL preferentially hydrolyzes PE compared to other phospholipids, and it preferentially releases DHA-containing lysophospholipids from the natural mixture of phospholipid molecular species.
2. Materials and Methods
2.1 Materials
Analytical grade chloroform, methanol, water and ammonia hydroxide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phospholipase D (Streptomyces sp) was obtained from BIOMED (Plymouth Meeting, PA, USA). Deuterated PC (16:0 (d31)-18:1 PC), 16:0 lysoPE, 17:0 lysoPC, 18:1 lysoPE and 18:1 lysoPC were from Avanti Polar Lipids (Alabaster, AL, USA). 14:0-14:0 PC, 14:0-14:0 PE, 16:0-16:0 PS, 16:0-16:0 PA and 16:0-22:6 PC, as well as L-serine and ethanolamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Silica gel (60–100 mesh) and Silica gel 60 plates were obtained from Analtech (Newark, DE, USA). Recombinant endothelial lipase, expressed in COS cells, was kindly provided by Dr. Daniel J. Rader, University of Pennsylvania. The specific activity of the enzyme preparation was 490 nmol fatty acid released/h/ml of medium, using labeled 16:0-16:0 PC substrate [23].
2.2 Purification of polyunsaturated fatty acid-containing PC
Polyunsaturated fatty acid-containing molecular species of PC used in the study were purified from the shark liver PC by HPLC using a silica column (5μm, 4.6 mm × 250 mm; ID, ZORBAX RX-Silica, Agilent, Apple Valley, MN, USA) as described previously [24]. Briefly, total lipids were extracted from shark liver with 40 volumes (v/v) of ethyl acetate/ethanol (2/1; v/v) while stirring at room temperature for 3 h [25]. After filtration and evaporation of the solvent under vacuum, the lipid extract was mixed with 30 volumes of acetone, and then the solution was stirred at 35°C for 1 h. The solution was filtered, and the filtrate was kept at −20 °C for 18 h. The precipitated phospholipids were collected again by filtration at −20 °C. This preparation contained 80 % PC, 15% lysoPC and 5 % of other lipids, as shown by thin-layer chromatography. The PC preparation was further purified by silica column chromatography using chloroform/methanol (50:50; v/v). The PC preparation thus obtained was used to prepare PUFA-enriched PC by silica HPLC, as well as for the preparation of PS, PE, and PA by transphosphatidylation.
2.3 Transphosphatidylation of phospholipids
Phospholipids with various polar head groups, but identical fatty acid composition were prepared by transphosphatidylation [26] of PC. Thus deuterated PS, PE, and PA were prepared by transphosphatidylation of 16:0(d31)-18:1 PC (25 mg) using phospholipase D (Streptomyces sp) (1 Unit). Similarly, phospholipid molecular species enriched in PUFAs (> 45% DHA-PC) were prepared from shark liver PC as described above. The molecular species composition of shark liver PC analyzed by mass spectrometry is shown in Table 1.
Table 1.
Molecular species composition of shark liver PC by LC/ESI-MS
| [M+H]+ | Total Carbons: Total Double Bonds | Mol. Species* | Percentage (%)** | |
|---|---|---|---|---|
| 718 | 32 | 1 | a14:0/18:1*** | 2.5 ± 0.5 |
| 732 | 32 | 1 | 14:0/18:1 | 2.2 ± 0.3 |
| 734 | 32 | 0 | 14:0/18:0 | 2.4 ± 0.2 |
| 16:0-16:0 | ||||
| 746 | 34 | 1 | a16:0/18:1 | 8.1 ± 1.2 |
| 760 | 34 | 1 | 16:0/18:1 | 6.6 ± 0.7 |
| 764 | 36 | 6 | a14:0/22:6 | 1.8 ± 0.4 |
| 766 | 36 | 5 | a14:0/22:5 | 1.5 ± 0.5 |
| 780 | 36 | 5 | 16:0/20:5 | 6.2 ± 0.7 |
| 782 | 36 | 4 | 16:0/20:4 | 8.2 ± 0.4 |
| 790 | 38 | 7 | p16:0/22:6*** | 3.6 ± 0.7 |
| 792 | 38 | 6 | a16:0/22:6 | 5.9 ± 0.5 |
| 794 | 38 | 5 | a18:1/20:4 | 2.1 ± 0.4 |
| a18:0/20:5 | ||||
| 806 | 38 | 6 | 16:0/22:6 | 21 ± 1.4 |
| 808 | 38 | 5 | 16:0/22:5 | 7.8 ± 0.8 |
| 18:0/20:5 | ||||
| 810 | 38 | 4 | 18:0/20:4 | 2.7 ± 0.4 |
| 818 | 40 | 7 | a18:1/22:6 | 2.3 ± 0.4 |
| 820 | 40 | 6 | a18:0/22:6 | 2.3 ± 0.3 |
| 822 | 40 | 5 | a18:0/22:5 | 2.5 ± 0.3 |
| 832 | 40 | 7 | 18:1/22:6 | 1.3 ± 0.2 |
| 834 | 40 | 6 | 18:0/22:6 | 7.5 ± 0.5 |
| 836 | 40 | 5 | 18:0/22:5 | 1.5 ± 0.4 |
sn-1 acyl (or alkenyl or alkyl) group/sn-2 acyl group
Semiquantitative results were generated from the average intensity of [M + H]+ ions of the molecular species of shark liver PC, based on three replicate analyses by LC/ESI/MS.
an ‘a’ preceding the name of the species indicates 1-alkyl-2acyl species, while a ‘p’ preceding the name indicates 1-alkenyl-2acyl species.
For a typical preparation of polyunsaturated PE, a mixture of 2 mL of acetate buffer (0.2M; pH 5.5), containing 40 mM of CaCL2 and 400 mg of ethanolamine, was prepared in a 20 mL glass disposable vial, and the pH was adjusted to 5.5 with glacial acetic acid. The mixture was added to another vial that contained 100 mg of shark liver PC. After the vial was flushed with argon, the reaction was started by adding 5 Units of phospholipase D (Streptomyces sp), and was carried out for 15 h in a final volume of 2 mL at 45 °C on a metabolic shaker. Total lipids were extracted with 20 mL of a mixture of chloroform/methanol 2/1 (v/v) and 2 mL of water. The upper phase was re-extracted with 10 mL of chloroform, and the combined lipid extracts were concentrated under nitrogen, and subjected to silica gel column chromatography. The PA (formed as by product in all preparations) was eluted with a mixture of chloroform/methanol (90/10, v/v), and the PE was eluted with a mixture of chloroform/methanol (70/30, v/v). The deuterium labeled substrates 16:0(d31)-18:1 PE and 16:0(d31)-18:1 PA were prepared from 16:0(d31)-18:1 PC, as described above, and the purity of these compounds was assessed by LC/MS (Fig. 1).
Figure 1.
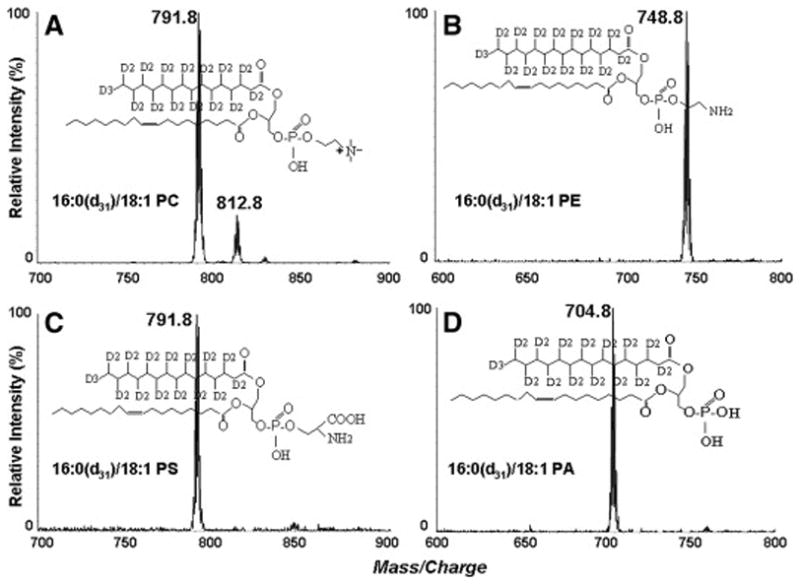
ESI-MS spectra of the deuterated phospholipid substrates (A) 16:0 (d31)-18:1 PC ([M+H]+, positive ion mode), (B) 16:0 (d31)-18:1 PE ([M+H]+, positive ion mode), (C) 16:0 (d31)-18:1 PS ([M-H]−, negative ion mode), and (D) 16:0 (d31)-18:1 PA ([M-H]−, negative ion mode). The PC ions (in A) at m/z 791.8 and m/z 812.8 are due to [M+H]+ and [M+Na]+, respectively
Identical procedures were followed for the preparation of DHA-enriched PS from the liver PC (Table 1), as well as deuterated PS from 16:0(d31)-18:1 PC, excepting that L-serine was used in the place of ethanolamine without acid adjustment. The positions occupied by 16:0(d31) and 18:1 chains in the deuterated phospholipids was verified by the negative ion ESI-MS/MS analysis of deprotonated molecule of 16:0(d31)-18:1 PS (spectrum not shown). The result showed that the intensity of the peak at m/z 286 (d3116:0), which is derived from the sn-1 position [27], is higher than that of the peak at m/z 281 (18:1), which is derived from the sn-2 position. Fragments at m/z 422, 440 and 417 correspond to [M - H - 87 - R2COOH]−, [M - H - 87 – R′2CO - H]− and [M - H - 87 - R1COOH]−, respectively.
2.4 Preparation of lipid substrates
The glycerol-stabilized emulsions of a quaternary mixture of equal amounts of 16:0(d31)-18:1 PC, 16:0(d31)-18:1 PE, 16:0(d31)-18:1 PS and 16:0(d31)-18:1 PA, were prepared as described [28] with a slight modification. The four deuterium-labeled lipids (500 μg each) in methanol/chloroform (2:1; v/v) were added into a test tube and taken to dryness under nitrogen. Cholesteryl oleate (36 mg) and glycerol (2 mL) were added to each tube, and the mixtures were sonicated on ice for 3 min followed by 1 min sonication at room temperature. The glycerol-stabilized emulsions of DHA-enriched natural phospholipids were prepared similarly.
2.5 Assay of enzyme activity and specificity
The reaction mixture contained 100 μl of the glycerol-stabilized phospholipids, 60 μl of water, 20 μl of Tris buffer (1 M), 20 μl of 15% bovine serum albumin and 200 μl of the EL preparation (conditioned medium) in a final volume of 400 μl. The reactions were started by the addition of the enzyme, and the samples were incubated at 37 °C for 30 min in a water bath. Total lipids were extracted by the method of Bligh and Dyer [29] after adding the internal standards, and were analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) and liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS), as described below.
2.6 Quantitative analyses of 16:0(d31)-18:1 phospholipids
LC/ESI-MS/MS analyses of the deuterium-labeled lipids were performed using a triple quadrupole instrument (PE/SCIEX LC/MS/MS 3000; Foster City, CA, USA) equipped with a Perkin Elmer HPLC system and an electrospray (ESI) ion source. The extracted lipids from controls and EL-treated samples were dissolved in 1 mL solvent (methanol/chloroform; 2:1; v/v). Internal standards of 14:0-14:0 PE, 14:0-14:0 PC, 16:0-16:0 PS, 16:0-16:0 PA, 16:0 lysoPE and 17:0 lysoPC, at concentrations of 2.5 μg/mL each for the diacyl phospholipids and 1.0 μg/mL each for lysophospholipids, were added before lipid extraction. LC analyses were performed using a silica HPLC column (3-μm, BETASIL Silica-100, 2.0 × 150 mm; Thermo, Foster City, CA. USA). Twenty μl of the samples were injected into the LC column, and the flow rate was maintained at 0.35 mL/min. The source temperature was set at 350 °C. The lipids were eluted with a linear gradient of 100% solvent A (chloroform/methanol/30% ammonium hydroxide, 80:19.5:0.5; v/v) to 100% solvent B (chloroform/methanol/water/30% ammonium hydroxide, 60:34.5:5:0.5; v/v) for 15 min, then in 100% solvent B for another 15 min [24]. Deuterium-labeled PE and PC species, as well as 18:1 lysoPE and 18:1 lysoPC species were quantitatively analyzed by the positive-ion neutral loss scan of 141 Da (for ethanolamine phospholipids), and the positive-ion precursor scan of 184 Da (for choline phospholipids). Collision energy was −28V for ethanolamine-containing lipids and −30V for choline-containing lipids [30,31]. Calibration curves for deuterated PC and PE were obtained by using 6 concentrations of the deuterated lipid (10, 20, 50, 100, 200, and 500 ng) and 50 ng of either 14:0-14:0 PE or 14:0-14:0 PC as internal standard. Similarly the calibration curves for lysoPE and lysoPC were obtained by using 5 concentrations of sn-1 18:1 lysoPE or sn-1 18:1 lysoPC (20, 50, 100, 200, and 500 ng) and either 20 ng of sn-1 16:0 lysoPE or 20 ng of sn-1 18:1 lysoPC as internal standard. All responses were linear within the above ranges with R2 values of 0.97 to 0.99. The [M + H]+ ions of the lipids were generated at the unit resolution, and intensities of the peaks were used to both plot the curves and calculate the concentrations of the lipids.
The deuterium-labeled PS species were quantitatively analyzed [31] using the negative-ion neutral loss scan of 87 Da, characteristic of deprotonated molecules of serine-containing lipids, using 16:0-16:0 PS (50 ng) as internal standard, while PA species were analyzed using precursor ion scan of 153 Da, using 16:0-16:0 PA (50 ng) as internal standard. Collision energy used was +30V and +45V for PS and PA, respectively. Linear calibration curves ranged from 50 to 500 ng for deuterium-labeled PS (y = 0.0034x + 0.2637; R2 = 0.95) and for deuterium-labeled PA (y = 0.0043x + 0.1456; R2 = 0.96).
2.7 Analyses of DHA-enriched species of PC, PE, PS and PA
Both the positive-ion LC/ESI-MS (for PC assay) and the negative ion LC/ESI-MS (for PE, PS and PA assay) were performed on a Micromass Platform LC/MS system (Micromass, Manchester, UK), equipped with a standard ESI source, and a quadrupole analyzer. Molecular species of the phospholipids and corresponding lysophospholipids were separated on a silica HPLC column as described above. The mass scanning range was from 350–1000 Da. Each sample of phospholipids was analyzed three times. LC/ESI-MS full-scan negative-ion mass spectra of PE, PS and PA and positive-ion mass spectra of PC (all averaged over the elution time) were used to determine the percentages of molecular species of the lipids in controls and EL-treated samples. The reproducibility of the analysis was within 15% for major components and better than 20% for minor species.
3. Results
3.1 Substrate selectivity of EL for phospholipids with various polar head groups
The substrate specificity of EL for polar head groups of the phospholipids was first studied using d31-labeled species of PC, PE, PS and PA with identical fatty acid composition. The mass spectra of the deuterated phospholipid substrates are shown in figure 1. Glycerol-stabilized quaternary mixtures containing equal amounts (25 μg for each) of 16:0(d31)-18:1 PC, 16:0(d31)-18:1 PE, 16:0(d31)-18:1 PS and 16:0(d31)-18:1 PA were incubated with buffer (controls) or 100 μl of EL preparation (activity: 490 nmol fatty acid released/h/ml) for 30 min, and the concentration of deuterium-labeled species were quantitatively analyzed by the positive-ion LC/ESI-MS/MS in the presence of internal standards. The loss of individual deuterium-labeled diacyl species is proportional to the substrate selectivity of EL for various phospholipids. Figure 2 shows representative mass spectra of 16:0 (d31)-18:1 PE and 16:0(d31)-18:1 PC extracted from both controls (Figs. 2A and 2C) and EL-treated samples (Figs. 2B and 2D). These results show that EL hydrolyzes preferentially PE compared to PC, with the hydrolysis rates of 53.4% and 36.6%, respectively (Fig. 3).
Figure 2.
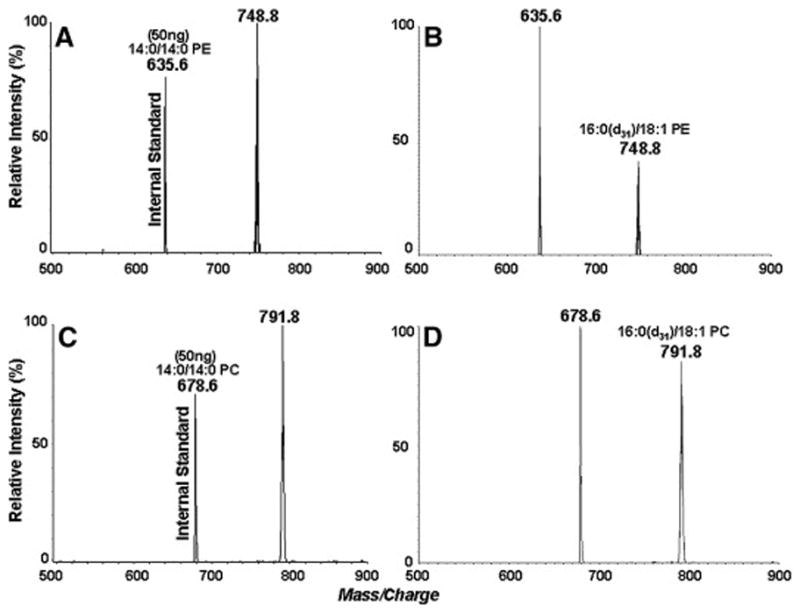
Phospholipid head group specificity of EL. An equimolar mixture of deuterated phospholipids of identical fatty acid composition (16:0-18:1) was treated with EL and the loss of each phospholipid was analyzed by LC/MS/MS.
A and B: Neutral loss (141 Da) positive ion scans of 16:0 (d31)-18:1 PE in control and in EL-treated sample respectively
C and D: Precursor ion scans (184-Da) of 16:0(d31)-18:1 PC in control and EL-treated samples respectively. 14:0-14:0 PE and 14:0-14:0 PC were used as internal standards for the quantitative analysis
Figure 3.
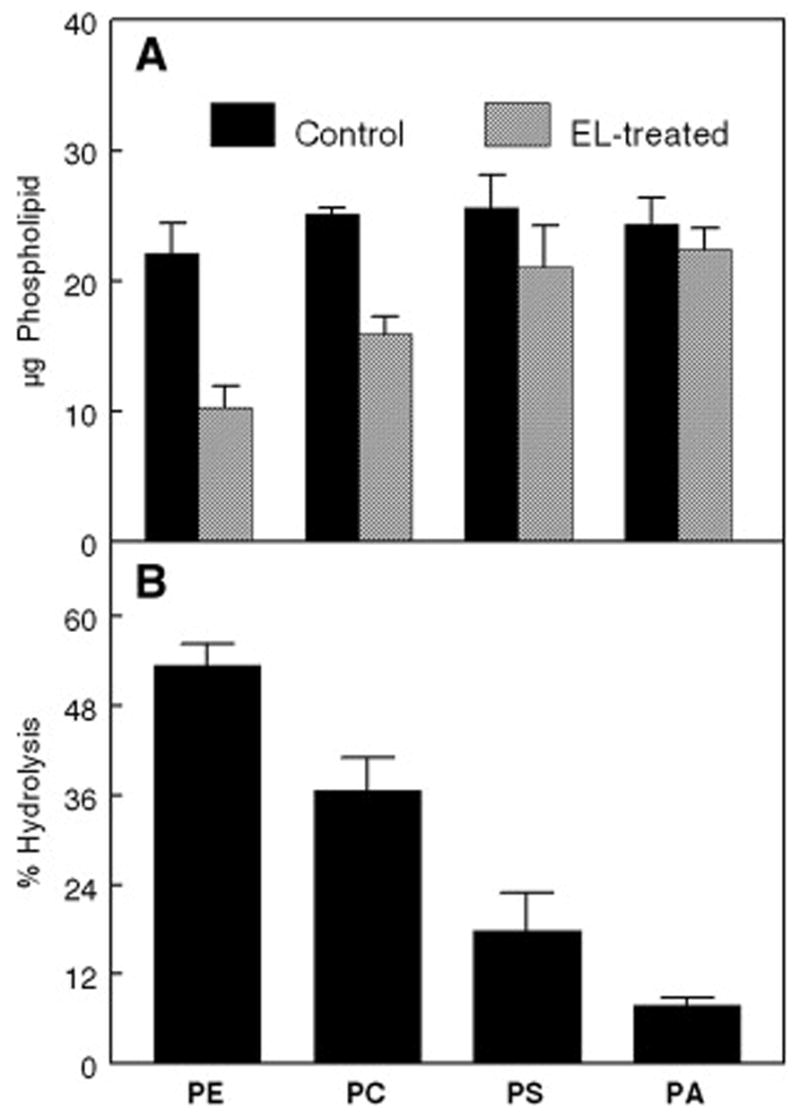
Phospholipid head group specificity. Values derived from the experiment described in Fig. 2.
A: Concentration of each phospholipid in control and EL-treated samples.
B: Percent of each phospholipid hydrolyzed by the enzyme.
The values shown are mean ± S.D of three determinations.
Identical procedures were followed for quantitative analyses of 16:0 (d31)-18:1 PS and 16:0 (d31)-18:1 PA species extracted from both controls and EL-treated samples (spectra not shown). Results indicated that EL prefers PS to PA, with the hydrolysis rates of 17.9% and 7.8%, respectively. Both PS and the PA are, however, poor substrates compared to PE and PC. Figure 3 shows the results obtained with the mixture of equal amounts of deuterium-labeled 16:0-18:1 substrates after 30-min incubation with EL. These results clearly show that the substrate specificity of EL is in the order: PE > PC > PS > PA.
The levels of sn-2-18:1 lysoPE and sn-2-18:1 lysoPC were also analyzed from the above samples, using sn-1-16:0 lysoPE and sn-1-17:0 lysoPC as internal standards (Figs. 4A and 4C). These results are in good agreement with those calculated from the loss of the deuterium-labeled diacyl phospholipids. The level of sn-2 18:1 lysoPE in EL-treated samples (Fig. 4B) is relatively higher than that of sn-2 18:1 lysoPC (Fig. 4D). There was no formation of 16:0 lysoPC (m/z 496) or 16:0 lysoPE (m/z 454) confirming the specificity of the enzyme for the sn-1 position. It should, however, be pointed out that quantitative analyses of sn-2-18:1 lysophospholipids could be affected by the lipid background in the low mass range (below 500 Da). The use of sn-1 lysophospholipids as both reference compounds and internal standards in quantitatively analyzing sn-2 lysophospholipids by mass spectrometry may also give rise to some error due to slight differences in peak intensities. These results, however, provided confirmatory data to support the conclusion that EL prefers PE over PC as substrate.
Figure 4.
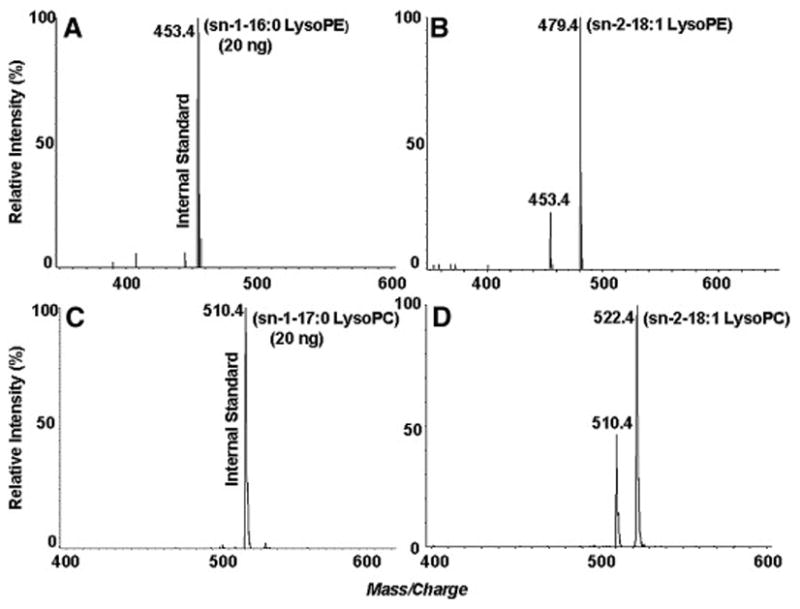
Quantitation of lysophospholipids generated by EL. The formation of sn-2-18:1 lyso PE was determined the neutral loss of 141 Da, using sn-1-16:0 lysoPE as the internal standard.
A: Control; B: EL-treated sample.
The formation of sn-2-18:1 lysoPC was determined by the 184 Da precursor ion scanning, using sn-1-17:0 lysoPC as the internal standard. C: Control; D: EL-treated sample.
3.2 Substrate specificity of EL for DHA-enriched species of phospholipids
To determine the substrate preference of EL among various phospholipids enriched in sn-2 DHA species, we prepared individual phospholipids containing similar fatty acid composition by transphosphatidylation of shark liver PC, followed by chromatographic purification. Although some differences in monounsaturated fatty acid percentages were still present because of the apparent preference of phospholipase D for certain PC species, all the phospholipids contained at least 45% of DHA-species, on the basis of mass spectrometry. The results show that DHA was present in about 56% of PC molecules, about 48% of PE molecules, about 54% of PS molecules, and about 46% of PA molecules (see also Tables 2, 3, 4 and 5). Although different phospholipid molecular species ionize to different extent, the method allows us to monitor the alteration of individual DHA-containing lipid molecule from multiple phospholipid substrates containing DHA. The structural identification of shark liver PC species will be described in detail elsewhere.
Table 2.
Percentages of DHA-enriched PC species after 30 min treatment by EL
| [M + H]+ | Molecular Speciesa | Control (%) | EL-treated (%) |
|---|---|---|---|
| 764.6 | a14:0/22:6* | 6.92 ± 1.2 | 8.28 ± 0.3 |
| 766.6 | a14:0/22:5 | 6.38 ± 1.1 | 11.3 ± 0.7 |
| 780.6 | 16:0/20:5** | 12.7 ± 0.5 | 6.22 ± 0.8 |
| 782.6 | 16:0/20:4 | 5.95 ± 0.2 | 3.10 ± 0.5 |
| 790.6 | a16:1/22:6 | 6.55 ± 0.5 | 7.55 ± 0.4 |
| 792.6 | a16:0/22:6 | 13.4 ± 1.4 | 26.1 ± 1.1 |
| 806.6 | 16:0/22:6 | 20.9 ± 0.5 | 13.2 ± 0.7 |
| 808.6 | 16:0/22:5 | 6.53 ± 0.3 | 3.72 ± 0.3 |
| 818.6 | a18:1/22:6 | 2.95 ± 0.6 | 3.44 ± 0.4 |
| 820.6 | a18:0/22:6 | 2.89 ± 0.8 | 2.84 ± 0.6 |
| 822.6 | a18:1/20:4 | 11.6 ± 0.9 | 12.4 ± 0.2 |
| 834.6 | 18:0/22:6 | 2.86 ± 0.4 | 1.85 ± 0.5 |
| Total | DHA-species | 56.5 |
All values mean ± S.D. of 3 analyses
an ‘a’ preceding the name of the species indicates 1-alkyl-2acyl species
Table 3.
Percentages of DHA-enriched PE species after 30 min treatment by EL
| [M−H]− | Molecular Species | Control (%) | EL-treated (%) |
|---|---|---|---|
| 702.6 | a16:0/18:1* | 4.89 ± 1.1 | 8.07 ± 0.2 |
| 716.6 | 16:0/18:1 | 8.54 ± 0.9 | 11.5 ± 0.5 |
| 736.6 | 16:0/20:5 | 5.12 ± 1.3 | 8.60 ± 0.3 |
| 738.6 | 16:0/20:4 | 10.4 ± 0.8 | 18.3 ± 0.8 |
| 748.6 | a16:0/22:6 | 4.83 ± 1.5 | 4.45 ± 0.6 |
| 750.6 | a16:0/22:5 | 3.96 ± 0.6 | 7.80 ± 1.1 |
| 762.6 | 16:0/22:6 | 23.7 ± 0.4 | 11.5 ± 0.7 |
| 764.6 | 16:0/22:5 | 14.6 ± 0.7 | 13.9 ± 1.3 |
| 774.6 | a18:1/22:6 | 3.87 ± 0.2 | 4.16 ± 0.8 |
| 790.6 | 18:0/22:6 | 15.6 ± 0.8 | 8.07 ± 1.0 |
| 792.6 | 18:0/22:5 | 4.49 ± 0.6 | 3.56 ± 0.7 |
| Total | DHA-species | 48.0 |
All values mean ± S.D. of 3 analyses
an ‘a’ preceding the name of the species indicates 1-alkyl-2acyl species
Table 4.
Percentages of DHA-enriched PS species after 30 min treatment by EL
| [M−H]− | Molecular Speciesa | Control (%) | EL-treated (%) |
|---|---|---|---|
| 746.6 | a16:0/18:1* | 3.41 ± 0.1 | 3.81 ± 0.2 |
| 760.6 | 16:0/18:1 | 4.29 ± 0.2 | 4.76 ± 0.6 |
| 780.6 | 16:0/20:5 | 5.89 ± 0.3 | 6.64 ± 0.2 |
| 782.6 | 16:0/20:4 | 8.83 ± 1.1 | 10.5 ± 0.6 |
| 792.6 | a16:0/22:6 | 6.56 ± 0.8 | 5.93 ± 0.4 |
| 794.6 | a16:0/22:5 | 8.08 ± 0.4 | 8.74 ± 1.2 |
| 806.6 | 16:0/22:6 | 29.9 ± 1.4 | 28.2 ± 1.7 |
| 808.6 | 16:0/22:5 | 9.50 ± 0.4 | 10.3 ± 0.5 |
| 820.6 | a18:0/22:6 | 4.59 ± 0.3 | 4.16 ± 0.3 |
| 834.6 | 18:0/22:6 | 13.2 ± 0.9 | 11.2 ± 0.7 |
| 836.6 | 18:0/22:5 | 5.74 ± 0.4 | 5.59 ± 0.5 |
| Total | DHA-species | 54.2 |
All values mean ± S.D. of 3 analyses
an ‘a’ preceding the name of the species indicates 1-alkyl-2acyl species
Table 5.
Percentages of DHA-enriched PA species after 30 min hydrolysis by EL
| [M−H]− | Molecular Species | Control (%) | EL-treated (%) |
|---|---|---|---|
| 659.6 | a16:0/18:1* | 5.01 ± 0.2 | 5.53 ± 0.8 |
| 673.6 | 16:0/18:1 | 4.15 ± 0.6 | 3.58 ± 0.2 |
| 693.6 | 16:0/20:5 | 4.02 ± 0.3 | 7.12 ± 0.4 |
| 695.6 | 16:0/20:4 | 12.0 ± 0.2 | 11.9 ± 1.6 |
| 705.6 | a16:0/22:6 | 4.50 ± 0.5 | 8.31 ± 0.3 |
| 707.6 | a16:0/22:5 | 8.99 ± 0.4 | 10.1 ± 1.5 |
| 719.6 | 16:0/22:6 | 26.3 ± 0.8 | 22.6 ± 0.7 |
| 721.6 | 16:0/22:5 | 15.0 ± 0.4 | 12.2 ± 0.8 |
| 733.6 | a18:0/22:6 | 3.09 ± 0.2 | 5.28 ± 0.6 |
| 747.6 | 18:0/22:6 | 12.2 ± 0.5 | 9.91 ± 0.5 |
| 749.6 | 18:0/22:5 | 4.74 ± 0.9 | 3.48 ± 0.6 |
| Total | DHA-species | 46.1 |
All values mean ± S.D. of 3 analyses
an ‘a’ preceding the name of the species indicates 1-alkyl-2acyl species
3.3 Substrate specificity of EL for glycerol-stabilized DHA-enriched PC species
Figure 5 shows positive ion LC/ESI-MS spectra of PC controls (A) and EL-treated PC samples (B). The largest decrease in percentages occurred for 16:0-22:6 and 16:0-20:5 species, whereas the percentages of the ether PCs increased showing their resistance to the EL hydrolysis (Table 2). These specificities were further confirmed by the appearance of lysoPC species after the reaction. These results clearly show an increase in lysoPC species containing 22:6 (m/z 568.4), 22:5 (m/z 570.4), 20:5 (m/z 542.4) and 20:4 (m/z 544.4)(Fig. 5C), while these species are absent in control sample (spectrum not shown).
Figure 5.
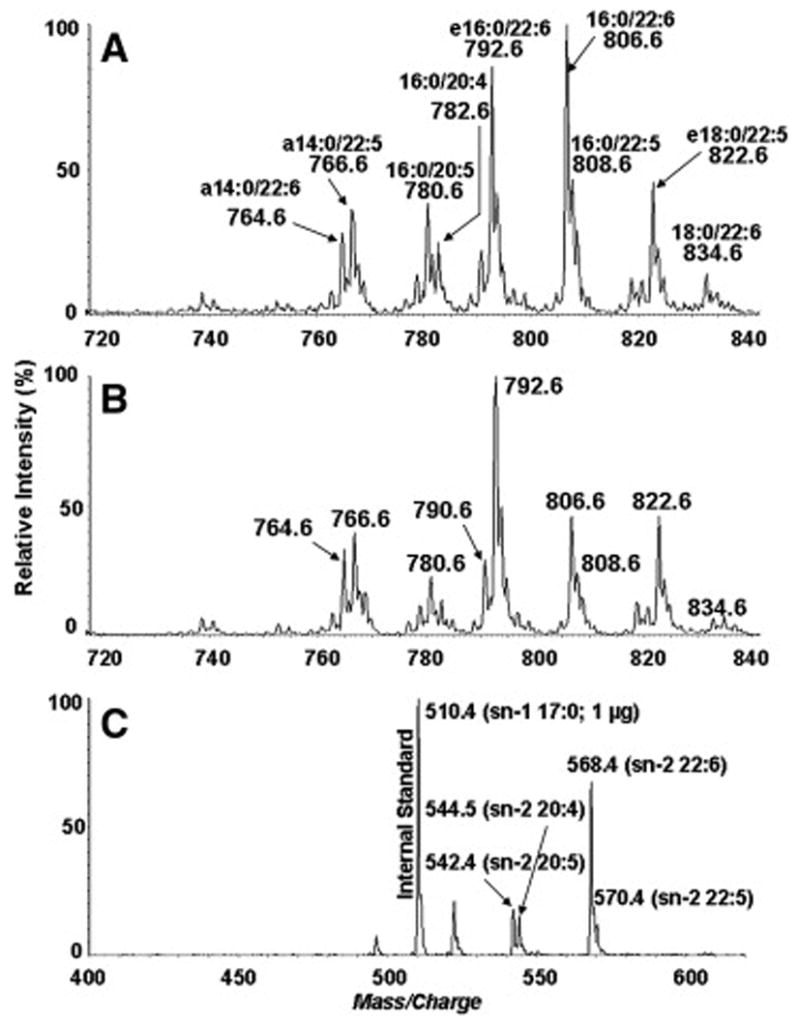
Substrate specificity of EL for DHA-enriched PC mixture. Positive-ion LC/ESI-MS profiles of HPLC-purified shark liver PC species extracted from control (A) and EL-treated sample (B). Figure 5C shows the spectrum of sn-2 lysoPC extracted from EL-treated sample
3.4 Substrate specificity of EL for DHA-enriched PE molecular species
Figure 6 shows the negative-ion LC/ESI-MS spectra of PE controls (A) and EL-treated PE samples (B). As shown in Table 3, the greatest decrease in percentages occurred for 16:0-22:6, 18:0-22:6, 16:0-22:5 and 18:0-22:5 species, whereas the greatest increases occurred for 16:0-20:4, 16:0-20:5 and ether PEs, as well as a slight increase for 16:0-18:1 PE, showing that the sn-2-22:6 and sn-2-22:5 species are preferred over the sn-2-20:4, sn-2-20:5 and sn-2-18:1 species. The sn-1 ether PEs were not hydrolyzed, as expected from the known positional specificity of the enzyme. These specificities were further confirmed by analyzing the lysoPE species formed after the enzyme reaction. There was a clear increase in the sn-2 22:6 lysoPE (m/z 524.5) and the sn-2 22:5 lysoPE (m/z 526.5)(Fig. 6C).
Figure 6.
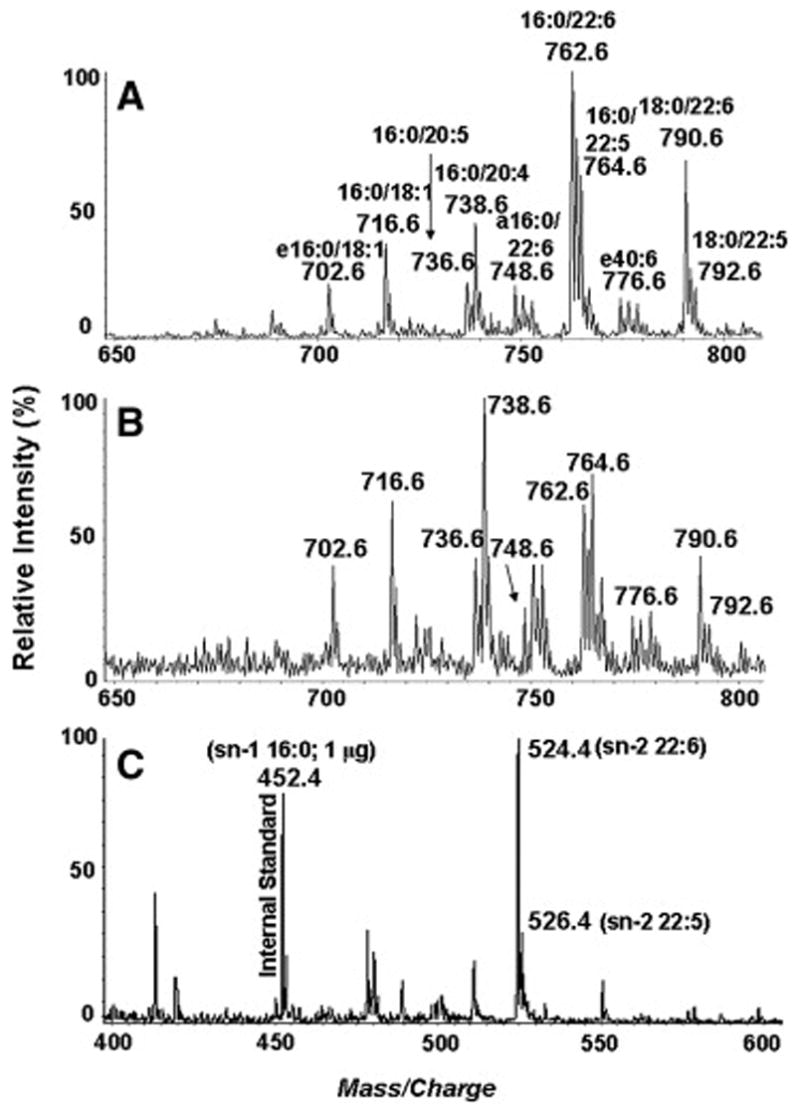
Substrate specificity of EL for DHA-enriched PE species. Negative-ion LC/ESI-MS profiles of transphosphatidylated shark liver PE extracted from control (A) and EL-treated sample (B). Figure 6C shows the spectrum of sn-2 lysoPE extracted from EL-treated sample.
3.5 Substrate specificity of EL for DHA-enriched PS and PA species
Compared to the PC and PE species, the hydrolysis of PS species by EL was much lower (Table 4). There was a significant decrease in the percentage of 18:0-22:6 and 18:0-22:5, as well as a slight decrease in 16:0-22:6 species. An increase in the percentage of 16:0-20:4 PS occurred, showing that this species is not hydrolyzed by EL. The poor hydrolysis of PS species is also confirmed by the generation of relatively smaller amounts of the sn-2 22:6 (m/z 568.4) and sn-2 22:5 (m/z 570.4) lysoPS species (spectra not shown).
Of the major species of PA, only 16:0-22:6 16:0-22:5, 18:0-22:6, and 18:0-22:5 species showed significant decreases, while there was increase in the percentages of 16:0-20:5, and the ether-PA species (Table 5). This result was confirmed by the low levels of the sn-2-lysoPA 22:6 (m/z 481) and 22:5 (m/z 483) produced after EL-treatment (spectra not shown).
4. Discussion
EL is the newest member of the lipase family that is expressed in several tissues including brain [21]. It is unique among the lipases in having a substrate preference predominantly for phospholipids, and in not requiring bile salts or apoproteins for its action on phospholipids. The activity of this enzyme has been shown to be inversely correlated with HDL levels in the plasma, showing its role in HDL metabolism [32,33]. The aim of the present study was to investigate its potential importance in the delivery of DHA to tissues such as the brain that are heavily dependent upon DHA and other polyunsaturated essential fatty acids. Previous studies have established the positional specificity of EL for the sn-1 ester bond of PC [20], although the fatty acid at the sn-2 position appears to strongly affect its activity [22]. Duong et al. [22] suggested that the presence of PUFA at sn-2 influences the binding of the enzyme to the lipid surface. In general, binding of the phospholipases to the lipid surface is regulated more by the surface charge (polar head groups) than by the acyl groups. Of the phospholipase activities present in plasma, secretory PLA2 (sPLA2) group IIa, is inactive against PC, but hydrolyzes PS and PA [34], while sPLA2 group V and group X hydrolyze both PE and PC efficiently [35]. Hepatic lipase, which also plays an important role in the hydrolysis of HDL phospholipids [36, 37], has been shown to hydrolyze PE more efficiently than PC [37, 38]. LCAT, which hydrolyzes more phospholipids than any other enzyme in plasma, hydrolyzes PC and PE equally, and has little activity towards PS and phosphatidylinositol [39]. It is therefore necessary to determine the effect of the polar head group on the phospholipid specificity of EL. Although the fatty acid specificity of the enzyme has been studied using PC species of defined fatty acid composition, the phospholipid head group specificity of EL has not been established. Using deuterium labeled lipid substrates of identical fatty acid composition (sn-1 16:0-sn-2 18:1), we show for the first time that PE is a better substrate for EL than PC, while PS and PA are poor substrates.
PE is a minor component of plasma phospholipids, comprising about 5% of total phospholipids [40, 41]. However, the PUFA content of plasma PE is greater than that of plasma PC [42]. Feeding omega-3 fatty acid to humans increases the DHA content of membrane PE more than that of membrane PC [42], and the administration of bovine brain PS significantly raises the level of plasma DHA-PE [43]. Therefore PE could be an important source of DHA for the brain, through the generation of DHA lysoPE by the action of EL. Quantitatively, however, since the lyso PC containing DHA would be the major product of the enzyme, the predominant vehicle for DHA delivery may still be lysoPC. It should also be pointed out that regardless of the polar head group, the preference of EL for phospholipid species containing sn-2 DHA is evident from our studies.
The fatty acid specificity of the enzyme for various molecular species of PC was determined using a natural mixture of PCs obtained from shark liver. For comparison purpose, we have also prepared PE, PS, and PA species of similar composition using the transphophatidylation reaction. It should be mentioned that the phospholipase D used here for the transphosphatidylation reaction appears to show some preference for the polyunsaturated species, and therefore the PE, PS, PA products were not of exactly the same fatty acid composition as the PC substrate. Nevertheless, the fatty acid specificity obtained with the natural mixtures of molecular species of phospholipids agrees with the studies of Duong et al. [22] who compared the activities obtained with rHDL containing single molecular species of PC. The use of mixture of natural phospholipids is important in understanding the physiological specificity of the enzyme, as well as for studying the efficacy of dietary supplements. Although shark liver PC contains relatively high percent of sn-1 ether-sn-2 DHA PC, the diester PCs containing DHA are preferred by the enzyme. Consequently, the DHA-lysoPC is the predominant product of the reaction.
Since the DHA-containing phospholipids are minor components of the lipoprotein phospholipids [44, 45], the selectivity of EL for the DHA-PC and DHA-PE may be important in the generation of lysoPC and lysoPE containing DHA, especially at the BBB. Studies by Bernoud et al. [11] showed a preferential transport of sn-2 DHA-lysoPC through the BBB in vitro. While no studies have been carried out with lysoPE, it is likely that this lipid is also transported in preference to free DHA across the BBB. Studies in whole animals also showed that the lysoPC species containing PUFAs are preferentially transported into brain as compared to the corresponding free fatty acids. These results therefore suggest a possible role for EL in the generation of DHA-lysoPC, and subsequent transport of DHA into the brain. Although Gauster et al. [20] reported that EL exhibits a weak lysophospholipase activity in prolonged incubations with native HDL3, we did not observe significant hydrolysis of lysoPC under the conditions of our assay, as evident from the good correlation between the loss of PC species and the appearance of lysoPC species.
The long term goal of our studies is to determine the efficacy of various DHA supplements in increasing the brain DHA concentration. Most of the supplements in the market are either triglycerides or ethyl esters of omega-3 fatty acid, and DHA from these supplements is incorporated predominantly into triglycerides, or into sn-2 position of phospholipids. Although small amounts of DHA can be transferred into the brain in the form of free fatty acid [46], DHA in the form of lysoPC appears to be more efficiently transported across the BBB [11–14]. Therefore, we investigated the possible mechanisms of the formation of DHA containing lysophospholipids. There are at least two enzymes in plasma that can generate 2-acyl DHA-lysoPC. One is LCAT, which is a modified phospholipase A that normally transfers the acyl group from the sn-2 position of PC to cholesterol [47]. This enzyme, however, transfers the sn-1 acyl group when the sn-2 position is occupied by DHA, possibly because of the limitation of the active site in accommodating DHA [44], thus generating DHA-lysoPC. The second is EL, which, as shown here, prefers DHA-containing phospholipids as substrates, and generates 2-acyl DHA lysoPC or lysoPE. The secretion of EL by the cells of BBB [21], and its substrate specificity for DHA phospholipids suggest that it may be an important enzyme for the transport of DHA into the brain. A third enzyme, hepatic lipase, also has the potential to generate DHA-lysoPC, but it appears to be specific for non-DHA PC species [22]. The presence of sn-1 and sn-2 DHA-lysoPC has been demonstrated in both human and rat plasma [48]. The relative importance of the two enzymes in this process is not clear. It would be of interest to know whether the transport of DHA into brain is impaired in the EL−/− mice.
Acknowledgments
These studies were supported in part by a grant from the National Institutes of Health, HL68585 (PVS). We thank Dr. Daniel J. Rader for providing the recombinant endothelial lipase.
Abbreviations used
- DHA
docosahexaenoic acid
- EL
endothelial lipase
- PUFA
polyunsaturated fatty acid
- BBB
blood-brain barrier
- LCAT
lecithin-cholesterol acyltransferase
- PLA1
phospho-lipase A1
- PLA2
phospholipase A2
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PA
phosphatidic acid
- lysoPC
lysophosphatidylcholine
- lysoPE
lysophosphatidylethanolamine
- lysoPS
lysophosphatidylserine
- lysoPA
lysophosphatidic acid
- 16:0 (d31)-18:1
1-hexadecanoyl (d31)-2-octadecenoyl
- 16:0 lysoPE
1-hexadecanoyl-2-hydroxy-glycero-sn-3-phosphoethanolamine
- 17:0 lysoPC
1-heptadecanoyl-2-hydroxy-glycero-sn-3-phosphocholine
- 18:1 lysoPE
1-octadecenoyl-2-hydroxy-glycero-sn-3-phosphoethanolamine
- 18:1 lysoPC
1-octadecenoyl-2-hydroxy-glycero-sn-3-phosphocholine
- 14:0-14:0 PC
1,2-ditetra-decanoyl-glycero-sn-3-phosphocholine
- 14:0-14:0 lysoPE
1,2-ditetradecanoyl-glycero-sn-3-phosphoethanolamine
- 16:0-16:0 PS
1,2-dihexadecanoyl-glycero-sn-3-phosphoserine
- 16:0-16:0 PS
1,2-dihexadecanoyl-glycero-sn-3-phosphoserine
- 16:0-16:0 PC
1,2-dihexadecanoyl-glycero-sn-3-phosphocholine
- 16:0-22:6
1-hexadecanoyl-2-docosahexaenoyl
- 18:0-22:6
1-octadecanoyl-2-docosahexaenoyl
- 16:0-22:5
1-hexadecanoyl-2-docosapentaenoyl
- 18:0-22:5
1-octadecanoyl-2-docosapentaenoyl
- 16:0-20:4
1-hexadecanoyl-2-arachidonoyl
- 16:0-20:5
1-hexadecanoyl-2-eicosapentaenoyl
- LC/ESI-MS/MS
liquid chromatography/electrospray ionization tandem mass spectrometry
- LC/ESI-MS
liquid chromatography/electrospray ionization mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glomset JA. Role of docosahexaenoic acid in neuronal plasma membranes. Sci STKE. 2006:p1–6. doi: 10.1126/stke.3212006pe6. [DOI] [PubMed] [Google Scholar]
- 2.Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 3.Catalan J, Toru M, Slotnick B, Murphy M, Greiner RS, Salem N. Cognitive deficits in docosahexaenoic acid-deficient rat. Behav Neurosci. 2002;116:1022–1031. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- 4.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onuki Y, Morishita M, Chiba Y, Tokiwa S, Takawama K. Docosahexaenoic acid and eicosapentaenoic acid induce changes in the physical properties of a lipid bilayer model membrane. Chem Pharm Bull. 2006;54:68–71. doi: 10.1248/cpb.54.68. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White ME, Frautschy A. Prevention of Alzheimer’s disease: Omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobio Aging. 2005;26S:S133–S136. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduced amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernoud N, Fenart L, Moliere P, Dehouck MP, Lagarde M, Cecchelli P, Lecerf J. Preferential transfer of 2-docosahexaenoyl-1-lysophosphatidylcholine through an in vitro blood-brain barrier over unesterified docosahexaenoic acid. J Neurochem. 1999;72:338–345. doi: 10.1046/j.1471-4159.1999.0720338.x. [DOI] [PubMed] [Google Scholar]
- 12.Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J. Lysophosphatidylcholine as preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci. 2001;16:201–204. doi: 10.1385/JMN:16:2-3:201. [DOI] [PubMed] [Google Scholar]
- 13.Thies F, Delachambre M, Bentejac M, Lagarde M, Lecerf J. Unsaturated fatty acids esterified in 2-acyl-1-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem. 1992;59:1110–1116. doi: 10.1111/j.1471-4159.1992.tb08353.x. [DOI] [PubMed] [Google Scholar]
- 14.Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC-DHA over unesterified DHA in the young rat brain. Am J Physiol. 1994;267:R1273–R1279. doi: 10.1152/ajpregu.1994.267.5.R1273. [DOI] [PubMed] [Google Scholar]
- 15.Subbaiah PV, Liu M, Bolan PJ, Paltauf F. Altered positional specificity of human plasma lecithin-cholesterol acyl-transferase in the presence of sn-2 arachidonoyl phosphatidylcholine. Mechanism of formation of saturated cholesteryl esters. Biochim Biophys Acta. 1992;345:83–92. doi: 10.1016/0005-2760(92)90261-s. [DOI] [PubMed] [Google Scholar]
- 16.Subbaiah PV, Sowa JM, Davidson MH. Evidence for altered positional specificity of LCAT in vivo: studies with docosahexaenoic acid feeding in humans. J Lipid Res. 2004;45:2245–2251. doi: 10.1194/jlr.M400197-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Brossard N, Croset M, Normand S, Pousin J, Lecerf J, Laville M, Tayot JL, Lagrade M. Human plasma albumin transport [13C]docosahexaenoic acid in two lipid forms to blood cells. J Lipid Res. 1997;38:1571–1582. [PubMed] [Google Scholar]
- 18.Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C] DHA in phosphatidylcholine. J Lipid Res. 1999;40:1867–1874. [PubMed] [Google Scholar]
- 19.Sekas G, Patton GM, Lincoln EC, Robins SJ. Origin of lysophosphatidylcholine; evidence for direct hepatic secretion in the rat. J Lab Clin Med. 1985;105:190–194. [PubMed] [Google Scholar]
- 20.Gauster M, Rechberger G, Sovic A, Horl G, Steyrer E, Sattler W, Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–1525. doi: 10.1194/jlr.M500054-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Sovic A, Panzenboeck U, Winterspergeer A, Kratzer I, Hammer A, Levak-Frank S, Frank S, Rader DJ, Malle E, Sattler W. Regulated expression of endothelial lipase by porcine brain capillary endothelial cells constituting the blood-brain barrier. J Neurochem. 2005;94:109–119. doi: 10.1111/j.1471-4159.2005.03175.x. [DOI] [PubMed] [Google Scholar]
- 22.Duong M, Psaltis M, Rader DJ, Marchadier D, Barter PJ, Rye KA. Evidence that hepatic lipase and endothelial lipase have different substrate specificity for high-density lipoprotein phospholipids. Biochemistry. 2003;42:13778–13785. doi: 10.1021/bi034990n. [DOI] [PubMed] [Google Scholar]
- 23.Chen YL, Xu Y. Isolation and quantitation of plasma lysophosphatidic acid by solid-phase extraction and capillary electrophoresis. Journal of Chromatography & Related Technologies. 2002;25:843–855. [Google Scholar]
- 24.Pruzanski W, Lambeau L, Lazdunsky M, Cho W, Kopilov J, Kuksis A. Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by group IIA, V and X secretory phospholipase A2. Biochim Biophys Acta. 2005;1736:38–50. doi: 10.1016/j.bbalip.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Lin JH, Liu LY, Yang MH, Lee MH. Ethyl acetate/ethyl alcohol mixtures as an alternative to Folch reagent for extracting animal lipids. J Agri Food Chem. 2004;52:4984–4986. doi: 10.1021/jf049360m. [DOI] [PubMed] [Google Scholar]
- 26.Comfurius P, Bevers EM, Zwaal RF. Enzymatic synthesis of phosphatidylserine on small scale by use of a one-phase system. J Lipid Res. 1990;31:1719–1721. [PubMed] [Google Scholar]
- 27.Chen S. Tandem mass spectrometric approach to determining structure of molecular species of aminophospholipids. Lipids. 1997;32:85–100. doi: 10.1007/s11745-997-0013-4. [DOI] [PubMed] [Google Scholar]
- 28.McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43:921–929. [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Gross RW. Electrospray ionization mass spectrometric analysis of human erythrocyte plasma membrane phospholipids. Proc Natl Acad Sci USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brugge B, Erben G, Sandhoff R, Wieland F, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen JC. Endothelial lipase: direct evidence for a role in HDL metabolism. J Clin Invest. 2003;111:318–321. doi: 10.1172/JCI17744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaye M, Lynch KJ, Marchadier KD, Maugeais C, Doan K, South V, Amin D, Perrpne M, Rader DJ. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21:424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 34.Snitko Y, Yoon ET, Cho W. High specificity of human secretory class II phospholipase A2 for phosphatidic acid. Biochem J. 1997;321:737–741. doi: 10.1042/bj3210737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 36.Connelly PW. The role of hepatic lipase in lipoprotein metabolism. Clin Chim Acta. 1999;286:243–255. doi: 10.1016/s0009-8981(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 37.Landin B, Nilsson A, Twu JS, Schotz MC. A role for hepatic lipase in chylomicron and high density lipoprotein phospholipid metabolism. J Lipid Res. 1984;25:559–563. [PubMed] [Google Scholar]
- 38.Landin B, Nilsson A. Metabolism of chylomicron phosphatidylethanolamine in the rat. Biochim Biophys Acta. 1984;794:105–113. doi: 10.1016/0005-2760(84)90058-4. [DOI] [PubMed] [Google Scholar]
- 39.Pownall HJ, Pao Q, Massey JB. Acyl chain and headgroup specificity of human plasma lecithin-cholersterol acyltransferase. Separation of matrix and molecular specificity. J Biol Chem. 1985;260:2146–2152. [PubMed] [Google Scholar]
- 40.Diagne A, Fauvel M, Record M, Chap H, Douste-Blazy L. Study on ether phospholipids: II Comparative composition of various tissues from human, rat and guinea pig. Biochim Biophy Acta. 1984;793:221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- 41.Phillips GB, Dodge JT. Composition of phospholipids and of phospholipid fatty acids in human plasma. J Lipid Res. 1967;8:676–681. [PubMed] [Google Scholar]
- 42.Popp-Snijders C, Schouten JA, van Blitterswijk WK, van der Veen EA. Changes in membrane lipid composition of human erythrocytes after dietary supplementation of n-3 polyunsaturated fatty acids. Maintenance of membrane fluidity. Biochim Biophys Acta. 1986;864:31–37. doi: 10.1016/0005-2736(86)90061-1. [DOI] [PubMed] [Google Scholar]
- 43.Palatini P, Viola G, Bigon R, Menegus A, Bruni A. Pharmacokinetic characterization of phosphatidylserine in the rat. Br J Pharmacol. 1991;102:345–350. doi: 10.1111/j.1476-5381.1991.tb12176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subbaiah PV, Liu M. Comparative studies on substrate specificity of lecithin-cholersterol acyltransferase towards the molecular species of phospholipids in plasma of 14 vertebrates. J Lipid Res. 1996;37:113–122. [PubMed] [Google Scholar]
- 45.Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci. 2001;16:159–165. doi: 10.1385/JMN:16:2-3:159. [DOI] [PubMed] [Google Scholar]
- 46.Rapoport SI, Chang CJMCJ, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- 47.Subbaiah PV, Liu M, Paltauf F. Role of sn-2 acyl group of phosphatidylcholine in determining the positional specificity of lecithin-cholesterol acyltransferase. Biochemistry. 1994;42:11533–11543. doi: 10.1021/bi00249a012. [DOI] [PubMed] [Google Scholar]
- 48.Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholine in human and rat. Biochem J. 1997;345:61–67. [PMC free article] [PubMed] [Google Scholar]


