Abstract
Eisosomes help sequester a subgroup of plasma membrane proteins into discrete membrane domains that colocalize with sites of endocytosis. Here we show that the major eisosome component Pil1 in vivo is a target of the long-chain base (LCB, the biosynthetic precursors to sphingolipids)-signaling pathway mediated by the Pkh-kinases. Eisosomes disassemble if Pil1 is hyperphosphorylated (i) upon overexpression of Pkh-kinases, (ii) upon reducing LCB concentrations by inhibiting serine-palmitoyl transferase in lcb1-mutant cells or by poisoning the enzyme with myriocin, and (iii) upon mimicking hyperphosphorylation in pil1-mutant cells. Conversely, more Pil1 assembles into eisosomes if Pil1 is hypophosphorylated (i) upon reducing Pkh-kinase activity in pkh1 pkh2-mutant cells, (ii) upon activating Pkh-kinases by addition of LCBs, and (iii) upon mimicking hypophosphorylation in pil1-mutant cells. The resulting enlarged eisosomes show altered organization. Other data suggest that Pkh signaling and sphingolipids are important for endocytosis. Taken together with our previous results that link eisosomes to endocytosis, these observations suggest that Pkh-kinase signaling relayed to Pil1 may help regulate endocytic events to modulate the organization of the plasma membrane.
Keywords: PDK1-kinases, Pil1, plasma membrane, sphingolipid signaling
Introduction
To perform its many functions, the composition of the plasma membrane is highly dynamic and is continually remodeled according to need. In yeast cells, several plasma membrane transporters and signaling proteins are expressed on the surface in a conditional, tightly regulated manner. This regulation is achieved by the interplay between the delivery of proteins to the plasma membrane and their retrieval by endocytosis. The yeast plasma membrane contains patches of segregated plasma membrane proteins that are thought to be the functional equivalents of the lipid raft domains of mammalian cells (Malinska et al, 2003, 2004; Opekarova et al, 2005; Grossmann et al, 2006). The functional relevance of these domains is so far unclear, but one intriguing possibility is that they provide the framework for efficient regulation of different plasma membrane proteins by segregating them into different pools that can be recruited into a specialized lipid/protein environment and can be taken up separately by endocytosis.
Recently large, immobile complexes that mark sites of endocytosis were discovered and termed eisosomes (Walther et al, 2006). Eisosomes are positioned underneath the plasma membrane. Their striking features include their uniform pattern at the plasma membrane, their stability over time, their relatively uniform size, and their composition of many copies of identical subunits. How these features are achieved molecularly is largely unknown; even our knowledge of the composition of eisosomes is still incomplete. The two major subunits of eisosomes are the Pil1 and Lsp1 proteins. Pil1 most likely is the main organizer of eisosomes, since its deletion leads to collapse of the normal eisosome organization and relocation of all other known eisosome components to a few eisosome remnants in the cell periphery (Walther et al, 2006; Grossmann et al, 2007). This effect is specific to Pil1, since deletion of the homologous Lsp1 has no such consequences and also does not aggravate the effect observed in yeast cells lacking Pil1.
Their uniform size, firm anchoring underneath the plasma membrane, and seemingly stable assembly into complexes that do not readily exchange subunits with a free cytoplasmic pool suggests that eisosomes, once assembled, are static structures. As eisosomes are constructed of a few thousand copies each of their two major protein subunits Pil1 and Lsp1, their structure must form as a repeating arrangement of many identical units. Such assembly suggests a role as a scaffolding device that may recruit other components and perhaps modulate their activities by concentrating them locally in a specialized lipid/protein environment. As such, eisosomes have emerged as central players in the organization of the plasma membrane, since deletion the PIL1 gene encoding one of their subunits leads to (i) large aberrant plasma membrane invaginations associated with eisosome remnants and (ii) loss of the normal domain distribution of several plasma membrane proteins and sterol lipids (Walther et al, 2006; Grossmann et al, 2007). The distinct subcompartments in the plasma membrane that they organize comprise small, randomly distributed membrane patches that colocalize with eisosomes.
Tanner and co-workers recently termed the eisosome-organized membrane domains MCCs for ‘Membrane Compartments occupied by Can1' (Grossmann et al, 2007), because they contain the arginine/proton symporter Can1, among other H+-gradient driven symporters and Sur7, a plasma membrane protein that is genetically linked to the endocytic machinery. As MCCs are enriched in sterols, they provide a unique lipid environment for the membrane proteins that are embedded in it. The domains are thought to be the equivalent of lipid-ordered domains in higher eukaryotes (Malinska et al, 2003, 2004). Deletion of Pil1 leads to a collapse of MCCs (Grossmann et al, 2007), suggesting that eisosomes are essential to determine their size and organization in the plasma membrane. Disruption of the H+ gradient across the plasma membrane also leads to release of Can1 and other transport proteins from MCCs, indicating that their localization of some membrane proteins can be dynamically controlled. Since functional studies suggest that their activity can strongly depend on the lipid environment (Lauwers and Andre, 2006), their recruitment into MCCs may provide an on/off switch. By contrast, other MCC constituents, such as Sur7, remain firmly anchored when the H+ gradient is disrupted and hence may serve structural roles that help define the membrane domain. Pil1 emerges as the main organizer, because its deletion causes the remaining eisosome and the plasma membrane proteins recruited there to disperse or collapse.
Pil1 and Lsp1 were initially characterized as modifiers of Pkh signaling (Zhang et al, 2004). The central components of Pkh -signaling are two redundant kinases Pkh1 and Pkh2 that are functional homologues of the mammalian phosphoinositide-dependent kinase (PDK1) (Casamayor et al, 1999). The salient features of this signaling pathway are conserved (Inagaki et al, 1999; Sun et al, 2000; Roelants et al, 2002). Several laboratories have shown that Pkh-kinases are required for efficient endocytosis (Friant et al, 2001; deHart et al, 2002). Endocytosis in yeast is mediated by actin patches (Engqvist-Goldstein and Drubin, 2003) and the defect in endocytosis of cells deficient in Pkh signaling correlates well with a decrease in polarization of the actin cytoskeleton in these cells (Daquinag et al, 2007; TC Walther, PS Aguilar, and P Walter, unpublished observation). Again, salient features of this signaling seem conserved, since the mammalian Pkh homologue PDK1 also regulates the actin cytoskeleton, for example during insulin signaling (Dong et al, 2000).
Even though the molecular events leading to activation of Pkh-kinases are still only poorly understood, we know that they are regulated by the long-chain bases (LCBs), phytosphingosine (PHS), and dihydrosphingosine (DHS) (Friant et al, 2001). Consistent with this finding, it was shown that LCBs modulate yeast endocytosis via Pkh-kinase regulation (Zanolari et al, 2000; Friant et al, 2001; deHart et al, 2002). At least part of this regulation might occur through eisosomes, since the major eisosome components Pil1 and Lsp1 are phosphorylated by Pkh-kinases in vitro (Zhang et al, 2004).
Several lines of evidence point to a functional connection between eisosomes and Pkh signaling: (i) genetic evidence suggest that the major eisosome components Pil1 and Lsp1 negatively regulate Pkh-kinases (Zhang et al, 2004); (ii) we and others biochemically found Pkh1 and Pkh2 associated with eisosomes (F Fröhlich, Ivan Mattic, Matthias Mann, and TC Walther, unpublished observation; Ho et al, 2002; Krogan et al, 2006); (iii) cellular localization of Pkh1 is reminiscent of eisosomes (Roelants et al, 2002); (iv) both eisosomes and Pkh-kinases have purported roles in endocytosis (Friant et al, 2001; deHart et al, 2002; Walther et al, 2006), and (v) Pkh-kinases phosphorylate Pil1 and Lsp1 in vitro (Zhang et al, 2004).
Here we investigated the effect of Pkh signaling on eisosomes and found that Pkh-kinases regulate aspects of eisosome assembly and organization. We suggest that this is part of a regulatory homeostasis mechanism that adjusts eisosome assembly in accordance to sphingolipid levels, perhaps providing negative feedback regulation to set eisosome abundance appropriately.
Results
Pkh-kinases localize to eisosomes
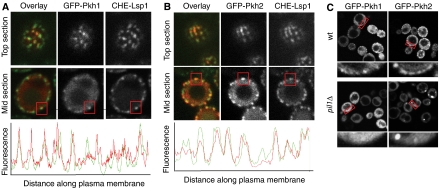
To further explore the connection of Pkh1 and Pkh2 with eisosomes, we first asked whether the Pkh-kinases associate with eisosomes in vivo. To this end, we expressed N-terminally GFP-tagged Pkh1 or Pkh2 in cells also expressing Cherry-tagged Lsp1 from its genomic locus. We found that the levels of GFP-Pkh1 and GFP-Pkh2 expressed from the PKH1 and PKH2 promoters were too low to detect the fusion proteins. We therefore overexpressed each fusion protein from the inducible GAL promoter, analyzing the earliest time points after induction that allowed reliable detection. As shown in Figure 1A, we observed robust induction of GFP fluorescence at a 1-h time point after shift from raffinose to galactose medium. Both GFP-Pkh1 and GFP-PKH2 localized to both a cytoplasmic pool and a distinct punctate pattern underlying the plasma membrane. For both kinases, the punctual staining colocalized with eisosomes marked by Lsp1-Cherry (Figure 1A and B). To get a quantitative impression of the degree of colocalization, we measured the relative fluorescence along the plasma membrane and overlayed the intensity profiles for either Pkh-kinase with the signal from Lsp1. For most eisosomes, we observed corresponding peaks of Pkh fluorescence (Figure 1A and B, lower panels).
Figure 1.
Pkh1 and Pkh2 are recruited to eisosomes. (A) 3GFP-Pkh1 expressed from the GAL promoter in a yeast strain expressing Lsp1-Cherry was imaged. Top- and mid-plane optical sections are shown for the single channel and an overlay in false colors (top panels). For midsections, the normalized intensity of the signal was plotted along a raced line underlying the cell surface (bottom panel). (B) Analogous analysis of Pkh2 localization. Boxes highlight colocalization of Pkh1 or Pkh2 with an individual eisosome. (C) Pil1 is required for localization of Pkh1 and Pkh2 to the plasma membrane. 3GFP-Pkh1 or 3GFP-Pkh2 was expressed from the GAL promoter either in wild-type cells (wt) or pil1Δ cells and imaged with a confocal microscope. Red boxes show area of the cell periphery that is shown in higher magnification below the corresponding image.
To test whether eisosomes are required for the targeting of Pkh-kinases to the plasma membrane, we expressed GFP-Pkh1 and GFP-Pkh2 in wild-type and pil1Δ cells, in which eisosomes are disrupted. Instead of being distributed evenly around the cell periphery, eisosome remnants cluster in pil1Δ cells to one or a few spots along the cell periphery. Indeed, pil1Δ cells also mislocalized Cherry-Pkh1 and Cherry-Pkh2 to the cytosol (Figure 1C, top panels) and to a few spots along the cell periphery, presumably corresponding to eisosome remnants. These results suggest that both Pkh1 and Pkh2 are at least partially localized to eisosomes, and that Pkh1 but not Pkh2 requires Pil1 for this association.
Sphingolipid signaling controls eisosome assembly
Pkh-kinases are regulated by LCBs, which are metabolic precursors of sphingolipids. LCBs may have roles as signaling molecules, transmitting information about cell stress and/or information about the lipid composition of the plasma membrane (Dickson et al, 1997). In vitro experiments have shown that Pil1 and Lsp1 can be phosphorylated by Pkh-kinases (Zhang et al, 2004), suggesting that eisosomes might be a target of the LCB-signaling pathway.
To test this hypothesis, we monitored the effects of altering sphingolipid synthesis on eisosome assembly and organization. To this end, we used a temperature-sensitive allele of LCB1 (lcb1-100), encoding serine-palmitoyl transferase, which is the rate-limiting enzyme in LCB synthesis. In lcb1-100 cells expressing Pil1-GFP, we observed a strong defect of eisosome assembly already at the permissive temperature (Figure 2A): cytoplasmic Pil1-GFP fluorescence was markedly increased compared with wild-type control cells. The effect is most clearly seen on a fluorescence profile of a line drawn through the diameter of the cell such that it bisects eisosomes, if visible, on either end (Figure 2A, lower panel). Accumulation of cytoplasmic Pil1-GFP was further aggravated after cells were shifted to the non-permissive temperature (Figure 2B). Under both permissive and non-permissive conditions, the total cellular Pil1-GFP levels were increased by roughly threefold (as assessed by western blotting, data not shown), perhaps due to a compensatory mechanism induced by failure to assemble eisosomes. To inhibit the synthesis of LCBs more acutely than possible with mutant cells that must be grown for many generations before analysis, we assessed the effects of myriocin, an inhibitor of Lcb1 (Fujita et al, 1994), on wild-type cells expressing Pil1-GFP. We imaged the cells 1 h after addition of myriocin (Figure 2C). Under these conditions the cellular Pil1 protein levels were unchanged (data not shown). The images show that cytoplasmic Pil1-GFP fluorescence increased and eisosome number at the cell periphery decreased. This experiment shows that LCB synthesis is required for maintenance of assembled eisosomes; due to the short time of myriocin treatment and the uniform phenotype observed, we can exclude that only newly formed eisosomes are affected by the drug.
Figure 2.
Sphingolipid signaling affects eisosome organization. (A) LCB1 is required for normal eisosome assembly and organization. Pil1-GFP was expressed in either wt cells (left panel) or cells harboring a mutation in LCB1 (lcb1-100). Cells were grown at 25°C and imaged with a confocal microscope. Representative middle sections are shown. Blue and red lines indicate the axis used for deriving the intensity profile plot shown below the images. (B) Elevated temperature aggravates the lcb1-100 phenotype. Cells were grown for 1.5 h at 37°C and analyzed as in panel A. (C) Myriocin addition affects eisosome assembly and organization. Myriocin (5 μM) (right panel) or buffer was added to wt cells expressing Pil1-GFP and imaged by confocal microscopy after 1.5 h incubation at 30°C. (D) PHS addition leads to increased eisosome assembly. C16 PHS (5 μM) or buffer was added to wt cells expressing Pil1-GFP, incubated for 1 h and imaged as in panel C. The relative size of eisosomes was measured on confocal images and averages are shown. Standard deviates from the mean are shown as error bars.
Since inhibiting LCB synthesis affects eisosomes, we reasoned that, conversely, an increase in LCBs may also affect their assembly and organization. To test this possibility, we added exogenous LCBs to the medium. Indeed, addition of 25 μM PHS for 1.5 h yielded eisosomes that are about twofold brighter in the fluorescence images (Figure 2D). In addition to the increased fluorescence per eisosome, eisosomes appear elongated in the fluorescent images, perhaps indicative of two eisosomes becoming stacked next to one another.
Similar to the effects of myriocin, addition of aureobasidin, which blocks the synthesis of ceramide downstream of LCBs, led to comparable defects in eisosome assembly and organization. Thus taken together, these data show that both LCBs and ceramides regulate the assembly of eisosomes: increased LCB levels lead to more Pil1 assembled into eisosomes, whereas decreased LCB or ceramide levels lead to less Pil1 assembled.
Pil1 phosphorylation depends on Pkh-kinases
Since Pkh-kinases can localize to eisosomes and Pil1 has been shown to be a Pkh substrate in vitro, we asked whether Pil1 is also phosphorylated in vivo by Pkh-kinases. Western blot analysis with an antibody specific to Pil1 showed that Pil1 migrates as a doublet on denaturing gels (Figure 3A; Supplementary Figure S1). The slower-migrating form represents a phosphorylated Pil1, since it was sensitive to λ-phosphatase treatment. Dephosphorylation of Pil1 was inhibited by a preincubation with phosphatase inhibitors (Figure 3A).
Figure 3.
Pil1 is phosphorylated in a Pkh1/2-dependent manner. (A) Pil1 is a phosphoprotein. Extracts of wt cells were either incubated with buffer (left lane), λ-phosphatase (middle lane), or λ-phosphatase after 5 min preincubation with a mixture of phosphatase inhibitors (right lane) for 30 min, and analyzed by western blot against Pil1. The lower panel shows the same blot reprobed with an antibody against 3-phosphoglycerate kinase 1 (Pgk1) used as loading control. (B) Phosphorylation of Pil1 depends on Pkh-kinases. Extracts from either Δpil1, wt, or pkh-ts cells grown at 25°C or shifted for 1.5 h to 37°C were analyzed as in panel A. (C) Overexpression of Pkh-kinases increases the levels of phosphorylated Pil1. Cells harboring PKH1 (pGAL-PKH1) or PKH2 (pGAL-PKH2) under the control of the GAL promoter were grown in raffinose-containing medium and shifted to galactose-containing medium for 1.5 h. Samples were analyzed as in panel A. (D) Ser273 is responsible for the shift in the mobility of phosphorylated Pil1 on SDS–PAGE gels. Extracts from cells either expressing wt Pil1-GFP or Pil1-GFP bearing a combination of seven amino acids mutated to alanine (S6, S45, S59, S230 T233, and S299, plus the residue indicated) were analyzed by western blot analysis. (E) A representative tandem MS spectrum is shown, in which threonine 233 of Pil1 is phosphorylated. Continuous sequence ions in the CID spectrum reveal the identity of the peptide, 222ALLELLDDSPVpTPGETRPAYDGYEASK248, and ions containing phosphate moiety (colored in red) indicate conclusively that Thr233 is phosphorylated. MS data for the remaining phosphorylation sites on Pil1 are provided in Supplementary Figures S2–S9. (F) Map of the identified phosphorylation sites on Pil1. A predicted central coiled-coil region is indicated in red.
We next asked whether Pil1 phosphorylation is dependent on Pkh-kinases. To this end, we examined the phosphorylation state of Pil1 in yeast cells bearing a temperature-sensitive allele of PKH1 and a deletion of PKH2 (Friant et al, 2001). Western blot analysis Pil1 of these strains showed that the phosphorylation of Pil1 is almost completely abolished under both permissive and restrictive conditions (Figure 3B). To further test the possibility that Pil1 is phosphorylated in a Pkh-kinase-dependent manner, we overexpressed Pkh1 and Pkh2 by placing each gene under control of the GAL promoter. At a 1.5-h time point after induction of either kinase, Pil1 phosphorylation was markedly increased (Figure 3C). Taken together our data establish Pil1 as a phosphoprotein that is a target of Pkh-kinase signaling in vivo.
To determine the sites of phosphorylation in Pil1, we partially purified Pil1 tagged with a tandem affinity purification tag or a myc-epitope and analyzed its phosphorylation by tandem mass spectrometry. This analysis revealed that Pil1 is phosphorylated at multiple sites. The MS/MS spectra of Pil1 showed phosphorylation of residues serines 16, 26, 45, 98, 163, 230, and 273, as well as of threonine 233 (a representative spectrum for this latter site is shown in Figure 3D; for the serine modifications see Supplementary Figures S2–S9). In addition, we found phosphorylation on serine 6 and serine 299, but these phosphorylated peptides were not detected in every preparation analyzed (F Chu and AL Burlingame, unpublished observation; Changhui Deng and Andrew Krutchinsky, personal communication). A large-scale analysis of the yeast phospho-proteome detected yet another phosphorylation site on serine 59 (Lyris de Godoy and Matthias Mann, personal communication). As shown in Figure 2E, the many phosphorylation sites on Pil1 are distributed across the protein, leaving a central predicted coiled-coil domain (K165-A198) unphosphorylated.
To determine which site(s) are responsible for the shift in mobility observed in SDS–PAGE gels (Figure 3A), we mutated all residues alone and in various combinations to alanine. Surprisingly mutation of up to six sites did alter Pil1's electrophoretic mobility. When we mutated S273 in addition, the full magnitude of the shift was abolished. Kinetic experiments dephosphorylating Pil1 in vitro by addition of phosphatases did not show intermediates in mobility (F Fröhlich and T Walther, unpublished observation), indicating that phosphorylation of serine 273 alone is responsible for the shift in mobility. As such, the gel shift assay does not report comprehensively on the phosphorylation status of Pil1.
Pkh signaling regulates eisosome assembly and organization
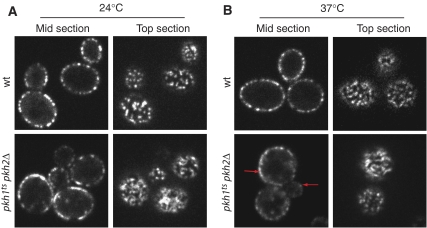
To address whether Pkh-kinase phosphorylation of Pil1 mediates the effect seen on eisosome assembly and organization upon inhibition of LCB synthesis and exogenously added LCBs, we next monitored the consequences of either inactivating or hyperactivating Pkh-kinases. First, we expressed Pil1-GFP in pkh1ts pkh2Δ cells (Friant et al, 2001). Figure 4 shows that even at the permissive temperature pkh1tspkh2Δ cells display increased fluorescence intensity at the cell membrane and an abnormal eisosome organization. As best seen in the optical top sections, Pil1-GFP is found in elongated filamentous structures that coalesce into a reticular pattern, rather than in discrete, uniform punctate characteristic of eisosomes in wild-type cells. This effect was further enhanced by shifting the cells to the restrictive temperature for 1 h, and was more prominent in larger mother cells (Figure 4B, lower panels; compare signal at red arrows). By contrast, eisosomes remained well defined and of uniform size in wild-type cells shifted to the elevated temperature (compare Figure 4A and B, top panels).
Figure 4.
Inactivation of Pkh-kinases leads to increased Pil1 assembly. (A) Pil1-GFP was expressed either in control wt cells or cells with a deletion in PKH2 and a temperature-sensitive allele of pkh1 (pkh1ts pkh2Δ). Cells were grown at 24°C and imaged by confocal microscopy. Representative optical midsections (left panels) or top sections (right panels) are shown. (B) Cells described in panel A were shifted to 37°C for 1.5 h and analyzed as described in panel A.
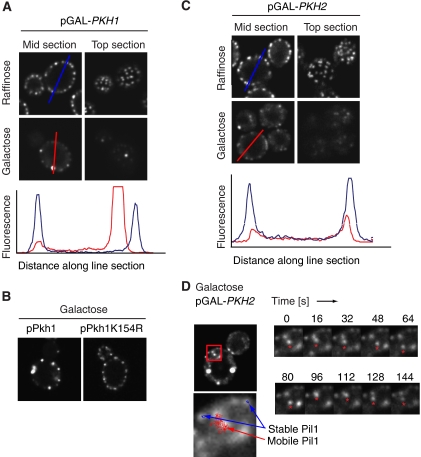
Second, we overexpressed Pkh1 and Pkh2 from the GAL promoter and monitored the localization of Pil1-GFP. The Pil1-GFP signal at the cell periphery rapidly decreased upon expression of either kinase. At a 1.5-h time point after induction only a few bright spots remained (Figure 5A and B, middle panels). This effect was specific to the enzymatic activity of Pkh-kinases, since overexpression of a kinase-dead mutant form of Pkh1, Pkh1(K154R) had no effect on eisosome assembly or organization (Figure 5B). The decrease of fluorescence signal from eisosomes at the cell periphery is most evident by comparing line plots through the diameter of the cell (Figure 5A and C, graphs). Since this reaction occurred relatively rapid and happened in all cells expressing the kinase, we conclude that this decrease of Pil1-GFP signal in the cell periphery must result from disassembly of existing eisosomes. Some cells continued to show normal eisosome organization, presumably due to loss of the overexpression plasmid.
Figure 5.
Overexpression of PKH-kinases leads to disassembly of eisosomes. (A) Pkh1 overexpression leads to eisosome disassembly. wt cells expressing Pil1-GFP and harboring PKH1 under the control of the GAL promoter were grown in non-inducing raffinose-containing medium and shifted 1.5 h to galactose. Cells from both conditions were analyzed by confocal microscopy and representative midsections (left panels) or top sections (right panels) are shown. The red and blue lines indicate the axis that was used to derive the intensity profile shown in the lower panel for the wt and Pkh1-expressing cells, respectively. (B) The effect of Pkh1 overexpression on eisosomes is dependent on the catalytic activity of the protein. Pkh1 was mutated in the catalytic lysine 154 and overexpressed as in panel A. Representative confocal midsections are shown. (C) Pkh2 overexpression leads to eisosome disassembly. An analogous experiment was performed with cells expressing Pkh2. (D) Pkh overexpression leads to mobilization of eisosomes. The experiment was performed as described in panel B and short time-lapse movies were recorded. The left panel shows an overview of the imaged cell and on the right a subset of the recorded frames is shown. The asterisks indicate a mobile cluster of Pil1. To follow movement of the Pil1 clusters, we tracked individual clusters over time (left bottom panel). A track of a mobile Pil1 cluster representing a 40 s movie is shown in red, and eisosomes that remained stable are shown in two blue tracks.
By contrast to eisosomes in wild-type cells, which are immobile, we frequently observed smaller Pil1-GFP foci that are detached from the plasma membrane in cells overexpressing either one of the Pkh-kinases. These structures were mobile within the cytoplasm, as apparent by time-lapse confocal microscopy (Figure 5D). We never observed mobile cytoplasmic Pil1-GFP foci in wild-type cells.
Pil1 phosphorylation can modulate its assembly into eisosomes
Taken together, the results presented so far show that (i) Pkh-kinases can localize to eisosomes, (ii) Pil1 phosphorylation is at least partially dependent on Pkh-kinases, and (iii) modulating Pkh-kinase activity leads to defects in eisosome assembly. To test whether the effect of Pkh-kinase activity on eisosome assembly and organization is directly due to phosphorylation of Pil1 rather than to an indirect effect, we next mutated the mapped phosphorylation sites to either alanine, which cannot be phosphorylated, or to negatively charged aspartate, which mimics phosphorylated serine or threonine. We introduced the mutated variants in the context of the PIL1-GFP fusion gene into yeast strains as the sole copy of PIL1. When analyzed by confocal fluorescence microscopy, none of the single mutant Pil1 variants showed a significant effect on eisosome assembly or organization (data not shown), including serine 273, the single residue responsible for the mobility shift on SDS–PAGE gels. Likewise, deletion of the whole C-terminus up to residue 266 had no effect on eisosome assembly or organization (TC Walther, PS Aguilar, and P Walter, unpublished data). We therefore mutated combinations of residues. Figure 6 shows the results of two quadruple mutants in which serines 45, 59, and 230, and threonine 233 were changed to alanine (Figure 6A) or aspartate (Figure 6B). In pil1(S45A,S59A,S230A,T233A)—or pil(4A) for short—cells, we found a strong increase in the fluorescence of eisosomes compared with wild-type controls and a structural defect qualitatively similar to but not quite as strong as the phenotype of the temperature-sensitive Pkh-kinase mutant cells (Figure 6A, lower panel). Conversely, in pil1(S45D,S59D,S230D,T233D)—or pil(4D) for short—cells, we observed mainly cytoplasmic Pil1-GFP with only a few bright spots remaining (Figure 6B, lower panel). This phenotype is reminiscent of that observed in cells overexpressing either of the Pkh-kinases (compare to Figure 5) and indicates that phosphorylation of these four residues is sufficient to exert this effect. The effect of this mutation on eisosome organization and assembly is not further aggravated by mutation of the Pkh-kinases (Figure 6C). To test whether these residues are also required for the Pkh-kinase effect on eisosomes, we expressed pil1(4A) in cells overexpressing Pkh1 or Pkh2. We found that Pil1(4A) is much more resistant to the disassembly and clustering observed for wild-type Pil1 under these conditions (Figure 6D).
Figure 6.
Phosphorylation of Pil1 regulates eisosome assembly. (A) Non-phosphorylatable Pil1 increases eisosome size. Pil1-GFP with serines 45, 59, 230, and threonine 233 mutated to alanine (pil1(4A)) was expressed as the only Pil1 and analyzed by confocal microscopy. Representative middle (left panels) and top sections (right panels) are shown. (B) Phospho-mimicking Pil1 mutants fail to assemble into eisosomes. Pil1 with the same residues indicated in panel A mutated to aspartate were analyzed as in panel A. (C) The pil1(4D) phenotype is dominant over the inactivation of Pkh-kinases. pil1(4D) was expressed in cells bearing a deletion in PKH2 and a temperature-sensitive allele of pkh1 (pkh1tspkh2Δ) grown at either 30°C (left panel) or 37°C (right panel). Representative confocal midsections are shown. (D) pil1(4A) is resistant to the effect of Pkh1 overexpression. wt Pil1-GFP (upper panels) and mutant Pil1(4A)-GFP (lower panels) were expressed in a cell with elevated Pkh1 levels expressed for 1.5 h form the GAL promoter in galactose-containing medium (see Figure 5A).
Together, these data suggest that phosphorylation of Pil1 by Pkh-kinases shifts the assembly equilibrium of Pil1 between a free, phosphorylated form and an eisosome-assembled, dephosphorylated form.
Discussion
Eisosomes help sequester a subgroup of plasma membrane proteins into discrete membrane domains that colocalize with sites of endocytosis (Walther et al, 2006; Grossmann et al, 2007). Here we show that the major eisosome component Pil1 in vivo is a target of the LCB-signaling pathway mediated by the Pkh-kinases. We found that Pkh-kinases can localize with eisosomes, consistent with other observations that suggest physically association based on high-throughput interaction screens and pull-down studies (Ho et al, 2002; Krogan et al, 2006). Moreover, it was shown previously that Pkh-kinases can phosphorylate Pil1 in vitro (Zhang et al, 2004). We find that phosphorylation is a critical regulator of Pil1 assembly into eisosomes and affects their organization. Eisosomes disassemble if Pil1 is hyperphosphorylated (i) upon overexpression of Pkh-kinases, (ii) upon reducing LCB concentrations by inhibiting serine-palmitoyl transferase in lcb1-mutant cells or by poisoning the enzyme with myriocin, and (iii) upon mimicking hyperphosphorylation in pil1(4D)-mutant cells. Conversely, more Pil1 assembles into eisosomes if Pil1 is hypophosphorylated (i) upon reducing Pkh-kinase activity in pkh1 pkh2-mutant cells, (ii) upon modulation of Pkh-kinases activity by addition of LCBs, and (iii) upon mimicking hypophosphorylation in pil1(4A)-mutant cells.
The resulting enlarged eisosomes show altered organization. Other data suggest that Pkh signaling and sphingolipids are important for endocytosis (Zanolari et al, 2000). Taken together with our previous results that link eisosomes to endocytosis (Walther et al, 2006), these observations suggest that Pkh-kinase signaling relayed to Pil1 may help regulate endocytic events.
At a first glance, the view of eisosomes as stable, uniformly sized entities is at odds with the assembly-disassembly of Pil1 upon phosphorylation reported here, as we show that the assembly and organizational properties of eisosomes are subject to modulation by phosphorylation. There are multiple possible resolutions to this paradox. First, newly made Pil1 may be phosphorylated and its dephosphorylation may accompany its assembly into eisosomes as part of their biosynthesis pathway. Our result that turning on Pkh-kinases dissembles pre-existing eisosomes argues against the notion that this is the exclusive role of the modification, although it does not rule it out conclusively. Second, an assembly/disassembly cycle of eisosomes may play role in their functional properties, yet be locally restricted so that under normal growth conditions only a few eisosomes in the cell are affected at any one time. This view would be consistent with our observation that endocytic events are restricted to a few active eisosomes at a time. Fluorescent photobleaching and recovery experiments may have missed the potentially dynamic behavior of only a few eisosomes. Third, the studies presented here may represent extreme end points of phosphorylation and dephosphorylation that do not truly reflect physiological conditions. The alternate actions of Pkh-kinases and cognate phosphatases under physiological conditions may only affect a subset of the total spectrum of potential phosphorylation sites on any particular Pil1 molecule, which could lead to local structural rearrangements within an eisosome without disrupting the complex as we observed upon extensive hyperphosphorylation. These possibilities are not mutually exclusive, and the currently available information does not allow us to distinguish between them.
A second paradox results from comparison of our results with previous data that showed an induction of Pkh activity by LCBs (Zanolari et al, 2000; Friant et al, 2001; Liu et al, 2005). A possible resolution comes from the observation that LCBs induce the phosphorylation of certain targets (such as Lsp1), whereas the activity toward Pil1 is decreased (Zhang et al, 2004). These data are in good agreement with our results suggesting that inhibition of sphingolipid synthesis results in increased activity of Pkh-kinases toward Pil1 and hyperphosphorylation of the protein.
Third, it is currently unknown why we observe one or a few large clusters of Pil1 under conditions were most eisosomes disassemble. One possibility is that the normal distribution of eisosomes breaks down under these conditions, leading to a clustering of molecules that would normally be part of eisosomes, such as Lsp1. This would then provide an abnormally high concentration of binding sites for Pil1 at these sites, which would in turn recruit some of the solubilized Pil1.
Our data suggest that the relative concentrations of LCBs and/or sphingolipids and ceramides are sensed via Pkh-kinases and transduced to Pil1 to regulate aspects of eisosome assembly and/or function. It is currently unknown how this sensing occurs at a molecular level. Since both inhibition of LCB and cermides synthesis have a similar effect on eisosomes, one possibility is that Pkh-kinases sense a property of the plasma membrane locally, perhaps in the membrane domain underlying eisosomes where those lipids are thought to be concentrated. Alternatively, and not mutually exclusive, LCBs or downstream metabolites may act as second messengers that bind the kinases and mediate the response of the Pkh-kinases. This is most likely part of a concerted cellular response to changing conditions that regulates endocytosis and adjusts the composition of the plasma membrane according to need.
Sphingolipids have been implicated in the regulation of many cellular processes such as cell growth, apoptosis, endocytosis, cell adhesion, and differentiation (Dickson, 1998; Futerman and Hannun, 2004; Dickson et al, 2006). In yeast, LCBs are massively increased during heat stress and the endocytic uptake of at least some highly abundant proteins, such as the uracil permease Fur4, is induced (Dickson et al, 1997; Jenkins et al, 1997; Bultynck et al, 2006). Moreover, Pkh-kinase-mediated regulation of endocytosis differentially affects the induced uptake of α-factor receptor Ste2 but not its constitutive recycling (Grosshans et al, 2006). Pkh-kinase regulation of endocytosis could thus potentially be used to selectively adjust the composition of the plasma membrane, suggesting that sphingolipid signaling might have a broad function in organizing the plasma membrane. Despite our astounding lack of knowledge regarding the regulation and physiological importance of this pathway, the potential of sphingolipid signaling as a drug target has already been demonstrated for myriocin, a compound that inhibits the generation of sphingolipids; myriocin was first characterized as an immune suppressive agent inducing apoptosis of cytotoxic T cells (Fujita et al, 1994). Such connections promise that expanding our most basic understanding of the mechanisms that organize the yeast plasma membrane may have profound implications for the physiology and pathology of mammalian cells.
Materials and methods
Yeast strains and plasmids
All yeast stains were generated in the W303 background. Lsp1-Cherry was introduced by homologous recombination using a PCR-based modification (Longtine et al, 1998) to generate the strain TWY566. The strain expressing Pil1-GFP was described in Walther et al (2006). PIL1-GFP was cloned into pRS306 and mutated using site-directed mutagenesis to generate plasmid pPIL4A (harboring substitutions S45A, S59A, S230A, T233A) or pPIL4D (harboring S45D, S59D, S230D, and T233D). Plasmids expressing seven mutations were generated in an analogous manner. Strains expressing either tagged or untagged versions of Pkh1 and Pkh2 were generated by transforming the following plasmids, which were generous gifts from Jeremy Thorner: for Pkh1 we transformed pAM73; for Pkh2, pAM79 (Casamayor et al, 1999) into a Pil1-GFP-expressing strain; for expression of 3GFP-Pkh1 or 3GFP-Pkh2, we transformed TWY566 with pFR37 or pER3, respectively (Roelants et al, 2002). pkh1tspkh2D strains were a generous gift from Howard Riezman (Friant et al, 2001).
Antibody generation
Antibodies against Pil1 were raised in rabbits against the full-length recombinant Pil1 protein expressed in Escherichia coli as a GST-fusion protein. The fusion protein was cleaved from the glutathione affinity column and further purified by ion-exchange chromatography on a MonoQ column. This protein was injected into rabbits in several boost cycles. Serum from rabbits was diluted 1:1000 for western blots.
Mapping of phosphorylation sites
For the analysis of Pil1 phosphorylation of Pil1, we froze cell from a 100 ml culture at OD=0.7 in 500 μl buffer (150 mM KoAc, 20 mM HEPES, pH 7.8, 1 mM MgAc) in liquid nitrogen. We extracted total protein by bead milling and subsequently clarified the extract by two consecutive spins of 4 min, 1000 g. Extracts were incubated with λ-phosphatase according to the manufacturer's instructions (NEB) in the presence or absence of a cocktail of phosphatase inhibitors (Sigma). After 30 min the reaction was stopped by addition of sample buffer. A 20 μg weight of total protein was run on a 10% SDS–PAGE and analyzed by western blot.
Affinity purification of Pil1 from 1 l of yeast culture expressing Pil1-myc was accomplished as previously described (Walther et al, 2006). Pil1-myc and eluted from beads with sample buffer (0.24 M Tris, 8% SDS, 1 mM β-mercaptoethanol, 40% glycerol, and 0.4% bromophenol blue) and loaded onto 4–20% SDS–PAGE Criterion Ready Gels (Bio-Rad). In-gel digestions on Pil1 bands were carried out utilizing a procedure described at http://msf.ucsf.edu/ingel.html. Typically, 100 ng of trypsin (porcine, side chain-protected; Promega, Madison, WI) was used for each gel band, and digestions were carried out at 37°C for 4 h. Peptides were extracted from gel pieces with 50 μl of 50% acetonitrile, 2% acetic acid three times, and the extraction solution was dried down to ∼10 μl. An aliquot of the digestion mixture was injected into an Ultimate capillary LC system via an FAMOS Autosampler (LC Packings, Sunnyvale, CA), and separated by a 75 μm × 15 cm reverse-phase capillary column at a flow rate of ∼330 nl/min. The HPLC eluent was fed directly into the micro-ion electrospray source of a QSTAR Pulsar QqTOF mass spectrometer (Applied Biosystem/MDS Sciex, Foster City, CA). Typical performance characteristics were >8000 resolution with 30 p.p.m. mass measurement accuracy in both MS and CID mode. LC-MS data were acquired in an information-dependent acquisition mode, cycling between 1-s MS acquisition followed by 3-s low-energy CID data acquisition. The centroided peak lists of the CID spectra were searched against the National Center for Biotechnology Information (NCBI) protein database using Batch-Tag, a program in the in-house version of the University of California San Francisco ProteinProspector package, considering phosphorylation on serine, threonine, and tyrosine as variable modifications. The CID spectra with putative phosphorylations were further inspected manually.
Microscopy
For fluorescence microscopy, yeast cells were grown to an OD=0.6 in either YPD or, when selecting for Pkh expression plasmids, in synthetic medium lacking leucine. Cells were mounted onto coverslips previously coated with concanavalin A and directly imaged either with a Zeiss LSM 510 confocal microscope or an ANDOR/TiLL iMIC spinning-disk confocal microscope. Images were processed using ImageJ software (www.rsb.info.nih.gov/ij/).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary Figure Legends
Acknowledgments
We thank Lyris de Godoy and Matthias Mann for sharing unpublished data, advice, and for providing excellent mass spectrometry infrastructure; Changhui Deng and Andrew Krutchinsky for sharing unpublished data and helpful discussions; Howard Riezman for the temperature-sensitive pkh-kinase-mutant strains; and Jeremy Thorner for the plasmids overexpressing Pkh-kinases. The UCSF mass spectrometry facility is supported by National Institutes of Health Grants RR 01614 (to ALB), RR 12961 (to ALB), and RR 15804 (to ALB). FC is a Rett Syndrome Research Foundation postdoctoral fellow. TCW thanks the Human Science Frontier Organization for funding through a long-term fellowship and a career development award. This work was supported by grants from the NIH to PW. PW is an Investigator of the Howard Hughes Medical Institute.
References
- Bultynck G, Heath VL, Majeed AP, Galan JM, Haguenauer-Tsapis R, Cyert MS (2006) Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol 26: 4729–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor A, Torrance PD, Kobayashi T, Thorner J, Alessi DR (1999) Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr Biol 9: 186–197 [DOI] [PubMed] [Google Scholar]
- Daquinag A, Fadri M, Jung SY, Qin J, Kunz J (2007) The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol 27: 633–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHart AK, Schnell JD, Allen DA, Hicke L (2002) The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J Cell Biol 156: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RC (1998) Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Ann Rev Biochem 67: 27–48 [DOI] [PubMed] [Google Scholar]
- Dickson RC, Nagiec EE, Skrzypek M, Tillman P, Wells GB, Lester RL (1997) Sphingolipids are potential heat stress signals in Saccharomyces. J Biol Chem 272: 30196–30200 [DOI] [PubMed] [Google Scholar]
- Dickson RC, Sumanasekera C, Lester RL (2006) Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog Lipid Res 45: 447–465 [DOI] [PubMed] [Google Scholar]
- Dong LQ, Landa LR, Wick MJ, Zhu L, Mukai H, Ono Y, Liu F (2000) Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc Natl Acad Sci USA 97: 5089–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG (2003) Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol 19: 287–332 [DOI] [PubMed] [Google Scholar]
- Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H (2001) Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J 20: 6783–6792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T (1994) Fungal metabolites Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot 47: 208–215 [DOI] [PubMed] [Google Scholar]
- Futerman AH, Hannun YA (2004) The complex life of simple sphingolipids. EMBO Rep 5: 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Grotsch H, Mukhopadhyay D, Fernandez IM, Pfannstiel J, Idrissi FZ, Lechner J, Riezman H, Geli MI (2006) TEDS site phosphorylation of the yeast myosins I is required for ligand-induced but not for constitutive endocytosis of the G protein-coupled receptor Ste2p. J Biol Chem 281: 11104–11114 [DOI] [PubMed] [Google Scholar]
- Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W (2007) Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 26: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann G, Opekarova M, Novakova L, Stolz J, Tanner W (2006) Lipid raft-based membrane compartmentation of a plant transport protein expressed in Saccharomyces cerevisiae. Eukaryot Cell 5: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C et al. (2002) Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415: 180–183 [DOI] [PubMed] [Google Scholar]
- Inagaki M, Schmelzle T, Yamaguchi K, Irie K, Hall MN, Matsumoto K (1999) PDK1 homologs activate the Pkc1–mitogen-activated protein kinase pathway in yeast. Mol Cell Biol 19: 8344–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y (1997) Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J Biol Chem 272: 32566–32572 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, Shales M, Zhang X, Davey M, Robinson MD, Paccanaro A, Bray JE, Sheung A, Beattie B et al. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643 [DOI] [PubMed] [Google Scholar]
- Lauwers E, Andre B (2006) Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic (Copenhagen, Denmark) 7: 1045–1059 [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang X, Lester RL, Dickson RC (2005) The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J Biol Chem 280: 22679–22687 [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast (Chichester, England) 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Malinska K, Malinsky J, Opekarova M, Tanner W (2003) Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 14: 4427–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinska K, Malinsky J, Opekarova M, Tanner W (2004) Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci 117: 6031–6041 [DOI] [PubMed] [Google Scholar]
- Opekarova M, Malinska K, Novakova L, Tanner W (2005) Differential effect of phosphatidylethanolamine depletion on raft proteins: further evidence for diversity of rafts in Saccharomyces cerevisiae. Biochim Biophys Acta 1711: 87–95 [DOI] [PubMed] [Google Scholar]
- Roelants FM, Torrance PD, Bezman N, Thorner J (2002) Pkh1 and pkh2 differentially phosphorylate and activate ypk1 and ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell 13: 3005–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, Fujita T, Kawasaki T, Kozutsumi Y (2000) Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol 20: 4411–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P (2006) Eisosomes mark static sites of endocytosis. Nature 439: 998–1003 [DOI] [PubMed] [Google Scholar]
- Zanolari B, Friant S, Funato K, Sutterlin C, Stevenson BJ, Riezman H (2000) Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J 19: 2824–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lester RL, Dickson RC (2004) Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J Biol Chem 279: 22030–22038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure S9
Supplementary Figure Legends