Abstract
In hepatocytes, Na+ influx through nonselective cation (NSC) channels represents a key point for regulation of cell volume. Under basal conditions, channels are closed, but both physiologic and pathologic stimuli lead to a large increase in Na+ and water influx. Since osmotic stimuli also activate mitogen-activated protein (MAP) kinase pathways, we have examined regulation of Na+ permeability and cell volume by MAP kinases in an HTC liver cell model. Under isotonic conditions, there was constitutive activity of p38 MAP kinase that was selectively inhibited by SB203580. Decreases in cell volume caused by hypertonic exposure had no effect on p38, but increases in cell volume caused by hypotonic exposure increased p38 activity tenfold. Na+ currents were small when cells were in isotonic media but could be increased by inhibiting constitutive p38 MAP kinase, thereby increasing cell volume. To evaluate the potential inhibitory role of p38 more directly, cells were dialyzed with recombinant p38α and its upstream activator, MEK-6, which substantially inhibited volume-sensitive currents. These findings indicate that constitutive p38 activity contributes to the low Na+ permeability necessary for maintenance of cell volume, and that recombinant p38 negatively modulates the set point for volume-sensitive channel opening. Thus, functional interactions between p38 MAP kinase and ion channels may represent an important target for modifying volume-sensitive liver functions.
Introduction
Mitogen-activated protein (MAP) kinases are ubiquitous serine/threonine protein kinases that play an important role in translating extracellular signals to the nucleus (1, 2). Among these, p38 MAP kinase represents a human homologue of the Saccharomyces cerevisiae HOG-1 gene product, a yeast MAP kinase required for cellular osmoregulation (2, 3). p38 MAP kinase is specifically regulated by changes in environmental osmolarity by dual tyrosine/threonine phosphorylation (4) mediated by two MAP kinase kinases (MEKs), MEK-3 and MEK-6 (5, 6). Members of the p38 signaling complex are also activated in response to lipopolysaccharides, proinflammatory cytokines, and ultraviolet radiation (4, 7, 8). It has been shown that p38 plays an important role in the cellular response to osmotic changes: inhibition of p38 by SB203580 prevents volume-sensitive induction of multiple mRNAs (2, 9, 10).
In addition to the effects on p38, change in cell volume also appears to be an important stimulus for modulation of other members of the MAP kinase family, including JNK and ERK (2, 9). Cell volume homeostasis is mandatory for maintenance of cellular integrity, but also represents a means of coupling changes in membrane transport to other organ-specific functions. An increase in cell volume, for example, appears to serve as a signal regulating many liver functions, stimulating protein synthesis, secretion, and gene expression. In addition to these physiologic roles, failure to regulate cell volume has been implicated in liver cell injury associated with alcohol, ischemia/reperfusion, and organ preservation (11–13). Thus, one function of MAP kinases may be to mediate volume-sensitive changes in gene expression.
Recent studies suggest that p38 MAP kinase is constitutively active in certain cells, and may also be capable of regulating membrane transport through direct effects on ion channels (14, 15). In hepatocytes, membrane Na+ permeability is low under basal conditions, but decreases in cell volume stimulate an adaptive response that involves Na+ influx through opening of nonselective cation (NSC) channels. The resulting water influx leads to restoration of cell volume toward basal values, a process referred to as regulatory volume increase (RVI) (16, 17). Since MAP kinases exhibit volume-sensitive changes in activity, the purpose of these studies was to assess the potential role of p38 and other MAP kinase signaling pathways in volume-dependent changes in membrane Na+ permeability. The findings suggest that constitutive activity of p38 MAP kinase plays an essential role in maintenance of cell volume through tonic inhibition of Na+-permeable ion channels.
Methods
Cell culture.
All studies were performed in HTC cells, a model rat hepatoma cell line that expresses ion channels and signaling pathways similar to those found in primary rat hepatocytes (18, 19). Decreases in HTC cell volume, stimulated by exposure to hypertonic buffer or oxidative stress, are followed by opening of Na+-permeable channels in the plasma membrane (20). Cells were passaged at weekly intervals and maintained in MEM containing HCO3– (Invitrogen, Grand Island, New York, USA) supplemented with 10% heat-inactivated FBS, L-glutamine (2 mM), penicillin (100 IU/ml), and streptomycin (100 μg/ml) as previously described (21).
Measurement of Na+ currents.
Membrane Na+ currents were measured using whole-cell patch-clamp techniques. Cells on a cover slip were mounted in a chamber (volume ∼400 μl) and perfused at 4–5 ml/min with a standard extracellular solution containing (in mM): 140 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 1 KH2PO4, 10 glucose, and 10 HEPES/NaOH (pH ∼7.40). The standard intracellular (pipette) solution for whole-cell recordings contained (in mM): 130 KCl, 10 NaCl, 2 MgCl2, 10 HEPES/KOH, 0.5 CaCl2, and 1 EGTA (pH 7.3), corresponding to a free [Ca2+] of approximately 100 nM (22). Patch pipettes were pulled from 7052 glass (Garner Glass Co., Claremont, California, USA) and had a resistance of 3–10 MΩ. Recordings were made with an Axopatch ID amplifier (Axon Instruments Inc., Foster City, California, USA), and were digitized (1 kHz) for storage on a computer and analyzed using pCLAMP version 6.0 (Axon Instruments Inc.) as previously described (23, 24). Three voltage protocols were used: (a) holding potential –40 mV, with 200-ms steps to 0 mV and –80 mV at 10-second intervals (for real-time tracings); (b) holding potential –40 mV, with 400-ms steps from –100 mV to +100 mV in 20-mV increments; and (c) holding potential –40 mV and voltage ramp from –100 mV to +100 mV over 200 ms. Current-voltage (I-V) relationships were generated from the “step” or “ramp” protocols as indicated. Pipette voltages are referred to the bath. In the whole-cell configuration, pipette voltage corresponds to the membrane potential, and upward deflections of the current trace indicate outward membrane current. Results are compared with control studies measured on the same day to minimize any effects of day-to-day variability, and are reported as current density (pA/pF) to normalize differences in cell size (21).
Cell size measurements.
Mean cell volume was measured in cell suspensions by electronic cell sizing with a Coulter Multisizer and AccuComp software version 1.19 (Coulter International Inc., Hialeah, Florida, USA) using an aperture of 100 μm, as previously described (25). Cells in subconfluent culture were harvested with 0.05% trypsin, suspended in cell culture media, centrifuged for 1 minute at approximately 1,000 g, resuspended in 3 ml of isotonic buffer, and incubated with gentle agitation for 30–45 minutes. Aliquots (∼500 μl) of cell suspension were added to 20 ml of isotonic or hypertonic (40% increase in NaCl or 100 mM sucrose, ∼395 mOsm) buffer. Measurements of approximately 20,000 cells were compared with basal values (time 0) at specified timepoints after exposure to isotonic or hypertonic buffer. Changes in value are expressed as relative volume normalized to the basal value. Experimental reagents were added as indicated.
MAP kinase activity.
Measurements of MAP kinase activity were performed as previously reported, with minor modifications (26, 27). Cells were exposed to hypotonic (100 mOsm) or hypertonic (∼500 mOsm) treatment for 5 minutes, then immediately homogenized in ice-cold lysis buffer (50 mM β-glycerophosphate (pH 7.2), 0.5% Triton X-100, 0.1 mM sodium vanadate, 2 mM MgCl2, 1 mM EGTA, 1 mM DTT, 2 μg/ml leupeptin, and 4 μg/ml aprotinin). The lysate was centrifuged at 4°C for 10 minutes at 10,000 g, and supernatants were adjusted to 100–200 μg protein in 0.5 ml.
For measurement of JNK activity, 100 μl of 10% GST–c-Jun agarose beads was added. After 2 hours of rocking incubation at 4°C, the adsorbed proteins were washed three times in lysis buffer and resuspended in 40 μl β-glycerophosphate (50 mM, pH 7.2), 0.1 mM sodium vanadate, 10 mM MgCl2, and 100 μM γ[32P]ATP (5,000 cpm/pmol). The reactions were incubated for 20 minutes at 30°C, then stopped by the addition of hot SDS sample buffer. The lysates were heated in a boiling water bath for 5 minutes and subsequently subjected to SDS-PAGE on a 10% polyacrylamide gel, followed by autoradiography. The bands corresponding to phosphorylated c-Jun were excised and counted in a liquid scintillation counter.
For measurement of ERK activity, 2.5 μl each of antisera against ERK1 and ERK2 (Sc-94 and Sc-93 rabbit polyclonal antisera; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) was added, along with 100 μl 10% protein A–Sepharose (Pharmacia Biotech Inc., Piscataway, New Jersey, USA). After 2 hours of rocking incubation at 4°C, the adsorbed proteins were washed three times in lysis buffer and resuspended in 40 μl β-glycerophosphate (50 mM, pH 7.2), 0.1 mM sodium vanadate, 10 mM MgCl2, 100 μM γ[32P]ATP (5,000 cpm/pmol), 50 μg/ml IP-20 (TTYADFIASGRTGRRNAIHD), and 200 μM EGF-R peptide (RRELVEPLTPSGEAPQALLR). The reactions were incubated for 20 minutes at 30°C, then stopped by the addition of 10 μl of 25% trichloroacetic acid. EGF-R peptide phosphorylation was assessed by phosphocellulose filter binding as described (28). PD98059 (100 μM), an inhibitor of MEK-1 (the upstream activator of ERK), was added in selected experiments.
For measurement of p38 MAP kinase activity, cultured cells were homogenized as above, and lysates were heated in a boiling water bath after addition of SDS sample buffer. Then 50 μg of protein was loaded per lane on either a 10% or a 12.5% polyacrylamide gel and subjected to SDS-PAGE. Proteins were transferred to either Immobilon or NitroPlus (Micron Separations Inc., Westboro, Massachusetts, USA), and the blot was blocked with 5% BSA, Fraction V (AMRESCO Inc., Solon, Ohio, USA) in Tris-buffered saline (pH 8) plus 0.1% Tween 80 for 2 hours at room temperature. Antisera incubation was done at 4°C for 16 hours in 5% BSA/Tween 80/TBS, after which the membrane was washed with Tween 80/TBS. Secondary antisera conjugated to horseradish peroxidase were incubated with the membrane for 2 hours at room temperature in 5% BSA/Tween 80/TBS, followed by washes as above and incubation with chemiluminescent substrate. An antibody specific for the activated (phosphorylated at Thr-180 and Tyr-182) form of p38 MAP kinase, and a control antibody that does not distinguish between phospho- and dephosphorylated p38 MAP kinase, were obtained from New England Biolabs Inc. (Beverly, Massachusetts, USA). Intensity of bands on Western blot films was determined by scanning with a video image scanner and digitizing software.
Reagents.
PD98059 (100 μM; Parke-Davis, Ann Arbor, Michigan, USA) was used as an inhibitor of MEK-1, the upstream activator of ERK (29). SB203580 (500 nM to 1 μM; Calbiochem-Novabiochem, La Jolla, California, USA) was used as an inhibitor of p38 (30, 31). In separate patch-clamp studies, recombinant p38α and MEK-6 were delivered to the cell interior by inclusion in the patch pipette (32). EGTA (5 mM) and amiloride (100 μM) were used in selected patch-clamp experiments.
Statistics.
Results are presented as mean ± SEM, with n representing the number of cells used for patch-clamp studies and the number of culture plates or repetitions for other assays. The Student paired or unpaired t test was used to assess statistical significance as indicated, and P values < 0.05 were considered to be statistically significant.
Results
Cell volume increases are mediated by channel-mediated Na+ influx.
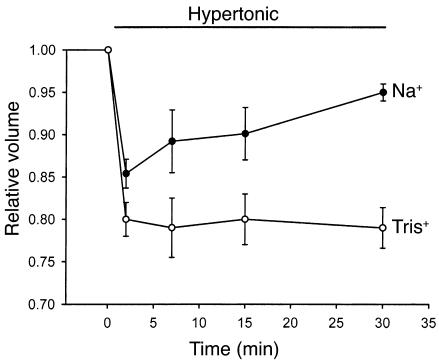
Exposure of cells to hypertonic buffer (40% increase in NaCl, ∼395 mOsm) caused a rapid initial decrease in relative volume to 0.85 ± 0.01 (n = 6, P < 0.001 compared with isotonic conditions) within 3 minutes. The decrease was followed by gradual recovery toward basal values (RVI) despite continued exposure to hypertonic buffer (0.94 ± 0.01 at 30 minutes; Figure 1). Volume recovery was inhibited (0.79 ± 0.02 at 30 minutes, P < 0.001) when Na+ in the bath solution was replaced with the impermeant ion Tris+, indicating that volume recovery is mediated by Na+ influx.
Figure 1.
Cell volume recovery is Na+ dependent. HTC cell suspensions were exposed to hypertonic buffer (40% increase in NaCl, ∼395 mOsm) at time 0, and cell volume (20,000 cells for each timepoint) was measured with a Coulter Multisizer. Hypertonic exposure resulted in a rapid initial decrease in cell volume, followed by gradual recovery toward basal values. When Na+ in the buffer solution was replaced with the impermeant cation Tris+, volume recovery was inhibited. Values represent mean ± SEM for four trials, with 20,000 cells for each timepoint.
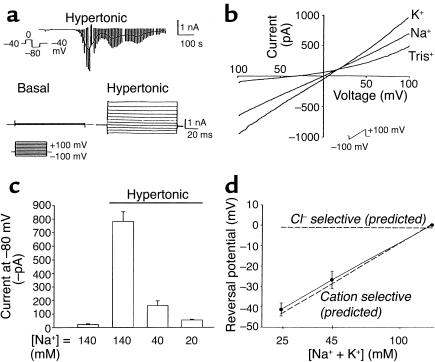
To determine if Na+ influx is mediated by a conductive pathway, parallel whole-cell patch-clamp studies were performed. With the standard bath and pipette solutions, the reversal potential for Na+ ions (ENa+) is +66 mV, and the reversal potential for cations (Ecat) is 0 mV. Consequently, the opening of a Na+-selective conductance would result in inward currents at both 0 mV and –80 mV, and the opening of an NSC conductance would result in inward currents at –80 mV but not at 0 mV (Ecat).
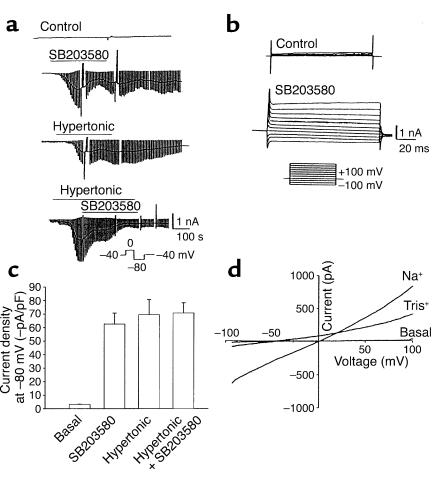
Under basal conditions, currents were small (less than –3.0 ± 1.1 pA/pF at –80 mV). Exposure to hypertonic buffer (20 mM sucrose, ∼320 mOsm) resulted in activation of inward currents within 2–3 minutes (representative trace shown in Figure 2a), increasing current density more than 30-fold, from –2.1 ± 0.3 pA/pF to –69.5 ± 11.2 pA/pF at –80 mV (P < 0.001, n = 20). Hypertonic-induced currents displayed a linear I-V relationship between –120 and +100 mV, and a reversal potential near 0 mV (Figure 2b). Because currents at –80 mV could be due to either Cl– or NSC conductance, several additional studies were performed to determine the permeability of the hypertonically stimulated conductance. First, the amplitude and reversal potential were unaffected by substitution of bath Na+ with K+ (–58.6 ± 13.1 pA/pF, n = 4; Figure 2b). Second, the Cl– channel blocker 5-nitro-2-(3-phenylpropylamine)benzoic acid (NPPB) had little effect on the magnitude of the currents at –80 mV (–55.4 ± 14.2, n = 4). Third, partial replacement of extracellular Na+ with Tris+ reduced inward currents and caused a negative shift in the reversal potential (Figure 2, b–d). Decreasing extracellular [Na+] to 40 mM decreased the magnitude of the inward currents (–160 ± 35.8 pA, n = 5, P < 0.001; Figure 2c) measured at –80 mV and shifted the reversal potential to –26.6 ± 2.3 mV (Figure 2d) compared with control (–781.1 ± 71.7 inward current pA, n = 9; reversal potential +0.5 ± 0.01 mV). Decreasing extracellular [Na+] to 20 mM decreased the magnitude of inward currents further (to –53.4 ± 6.17 pA, n = 4, P < 0.001), and shifted the reversal potential in a more negative direction (to –41.3 ± 3.8 mV). It should be noted that while substitution with Tris+ decreased inward currents as expected for a cation-selective channel, Tris+ also decreased the magnitude of outward currents measured at positive voltage potentials (Figure 2b), suggesting that Tris+ may also display nonspecific channel-blocking properties. The shift in reversal potential at the different extracellular cation concentrations is shown in Figure 2d. As shown, the findings are consistent with the reversal potential predicted by the Goldman-Hodgkin-Katz equation for a primary cation conductance (dotted line). The predicted reversal potential for a primary Cl– conductance is shown for comparison. Taken together, these findings are consistent with volume-sensitive Na+ influx through opening of an NSC channel with approximately equal Na+ and K+ permeability.
Figure 2.
Hypertonic exposure activates NSC currents. (a) Representative whole-cell recording. Currents at –80 mV (downward deflection of the current tracing) correspond to INa+ (see Methods). Hypertonic exposure (sucrose 20 mM, ∼320 mOsm) resulted in activation of inward currents (top tracing). A voltage-step protocol (test potentials between –100 mV and +100 mV in 20-mV increments) was used to measure basal and hypertonic-induced currents (bottom tracings). (b) I-V relationship of whole-cell currents. Currents induced by hypertonic exposure were measured utilizing a voltage-ramp protocol (–100 mV to +100 mV over 200 ms). Hypertonicity increased current amplitude with either Na+ or K+ as the primary extracellular cation, characterized by reversal near 0 mV. When Na+ in the extracellular solution was partially replaced with the impermeant cation Tris+ (final [Na+], 40 mM), there was a significant decrease in inward current amplitude, and a shift in reversal potential to –26.6 ± 2.3 mV (n = 4), as expected for a cation-selective channel. (c) Decreasing [Na+] in the extracellular solution by partial replacement with the impermeant cation Tris+ resulted in a significant decrease in the magnitude of the inward currents measured at –80 mV. (d) Decreasing [Na+ + K+], by replacement with Tris+, resulted in a shift in reversal potential consistent with the predicted reversal potential for a cation-selective conductance (shown by dotted line). The expected reversal potential for a primary Cl– conductance is shown by the dotted line at top.
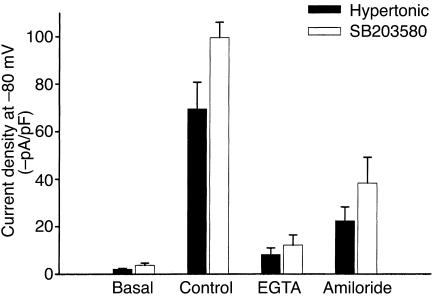
HTC cells have previously been shown to express an NSC conductance that displays Ca2+-dependent opening (18, 20). To determine if this hypertonically stimulated cation conductance is Ca2+ dependent, separate experiments were performed with low intracellular [Ca2+]. Increasing EGTA (5 mM, no added Ca2+) in the pipette solution inhibited current activation by hypertonic exposure (–8.1 ± 2.8 pA/pF, n = 5, P < 0.001; see Figure 5, solid bars) compared with control conditions (free [Ca2+] ∼100 nM, –69.5 ± 11.2 pA/pF, n = 20). Previous studies of primary rat hepatocytes indicated that RVI following hypertonic exposure is mediated by an amiloride-sensitive Na+ conductance (16, 33). To determine whether the hypertonically stimulated conductance in HTC cells displays similar properties, studies were performed in the presence or absence of the Na+ channel inhibitor amiloride (100 μM). Amiloride inhibited hypertonically stimulated (50 mM sucrose) currents by approximately 68% (–37.7 ± 5.8 pA/pF, n = 5, P < 0.01) compared with control (–116.9 ± 17.8 pA/pF, n = 9; see Figure 5, solid bars).
Figure 5.
Comparison of hypertonic- and SB203580-induced currents. Average current density for currents activated by hypertonic exposure (20 mM sucrose) or SB203580 (500 nM) was measured at –80 mV. Both hypertonic- and SB203580-induced currents were inhibited by inclusion of EGTA (5 mM, no added Ca2+) in the patch pipette solution, and by amiloride (100 μM) in the extracellular solution (P < 0.01).
Osmolarity-sensitive changes in MAP kinase activity.
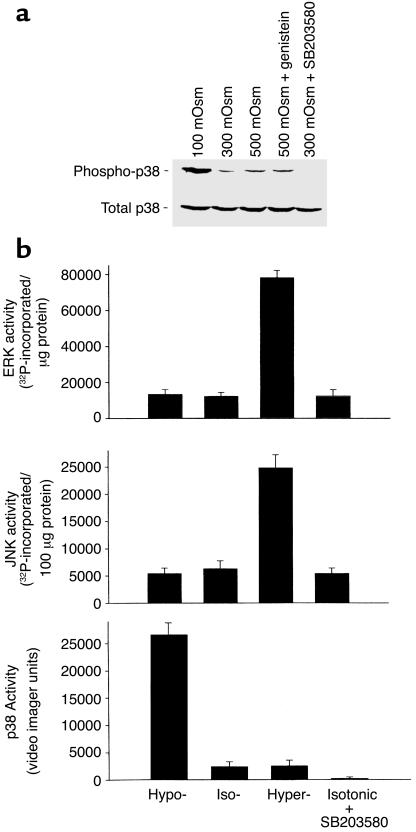
To evaluate the effects of HTC cell size on MAP kinase activity, cells were exposed to hypertonic (500 mOsm) or hypotonic (100 mOsm) conditions for 5 minutes, and kinase activity was assessed. Representative measurements of p38 MAP kinase are shown in Figure 3a; summary data for all kinases are shown in Figure 3b. Under isotonic conditions, there was detectable constitutive activity of p38, JNK, and ERK kinases. Decreases in cell volume caused by hypertonic exposure increase in both ERK (fivefold) and JNK (fourfold) activity, but had little effect on p38 MAP kinase activity. In separate studies, the MEK-1 inhibitor PD98059 (100 μM) inhibited both basal (by 65.5% ± 10.1%) and hypertonically induced (by 86.1% ± 15.5%) ERK activity (data not shown).
Figure 3.
Osmolarity-sensitive changes in MAP kinase activity. HTC cells were exposed to isotonic (∼300 mOsm), hypotonic (∼100 mOsm), or hypertonic (∼500 mOsm) buffer solutions for 5 minutes, then immediately homogenized in lysis buffer. (a) Cell lysates were subjected to SDS-PAGE and Western blot analysis with either an antibody specific for the activated (phosphorylated at Thr-180 and Tyr-182) form of p38 (phospho-p38) or a control antibody that does not distinguish between phospho- and dephosphorylated p38 MAP kinase (total p38). Constitutive phospho-p38 activity was observed under isotonic conditions; values increased with hypotonic exposure. Activity was inhibited by the p38 inhibitor SB203580 (1 μM), but not by the tyrosine kinase inhibitor genistein (10 μM). None of the various exposures resulted in changes in total p38. (b) Average MAP kinase activity in response to osmotic changes performed as described in Methods. Under isotonic conditions, there was constitutive activity of p38, JNK, and ERK kinases. Hypertonic exposure stimulated a large increase in both ERK (fivefold) and JNK (fourfold) activity, but had little effect on p38 MAP kinase activity. In contrast, hypotonic exposure resulted in a large increase in p38 MAP kinase activity (tenfold), but had little effect on ERK or JNK activity compared with isotonic conditions. The putative p38 MAP kinase inhibitor SB203580 did not affect ERK or JNK activity, but completely inhibited constitutive p38 MAP kinase activity.
In contrast, increases in cell volume caused by hypotonic exposure resulted in a large increase in p38 MAP kinase activity (tenfold), but had little effect on ERK or JNK activity compared with isotonic conditions. The putative p38 MAP kinase inhibitor SB203580 (1 μM) did not effect ERK or JNK activity, but completely inhibited both constitutive and volume-sensitive p38 MAP kinase activity (Figure 3). These studies demonstrate that (a) there is constitutive ERK, JNK, and p38 MAP kinase activity under basal (isotonic) conditions; (b) increases in cell volume (hypotonic exposure) increase p38 activity; and (c) there is differential regulation of p38 MAP kinase activity versus ERK and JNK activity in response to changes in HTC cell volume.
Na+ permeability is not influenced by ERK activity.
Given the large increase in ERK activity with hypertonic exposure, the potential role of ERK in NSC channel regulation was evaluated. If ERK modulates volume-sensitive membrane Na+ permeability through MEK-1 activation, inhibition of MEK-1 would be expected to prevent channel opening. To test this hypothesis, whole-cell patch-clamp experiments were performed in the presence or absence of PD98059 (100 μM), an inhibitor of MEK-1. In control cells, exposure to hypertonic buffer (50 mM sucrose, ∼350 mOsm) resulted in characteristic increases in Na+ permeability (–86.1 ± 14.2 pA/pF, n = 6), and the response was unchanged in the presence of PD98059 (–68.9 ± 9.3 pA/pF, n = 5, data not shown). Thus, volume-sensitive increases in ERK activity are not likely to be involved in the regulation of Na+ influx.
Inhibition of p38 increases membrane Na+ permeability.
Since there did not appear to be a direct effect of ERK on channel regulation, the alternative possibility that p38 MAP kinase has an inhibitory role in channel regulation was explored by measuring membrane Na+ permeability under basal conditions and during inhibition of p38 (Figure 4). Whole-cell currents were small in control cells (–2.1 ± 0.3 pA/pF, n = 15). However, exposure to the p38 MAP kinase inhibitor SB203580 (500 nM) was followed by an increase in membrane Na+ permeability in the absence of a volume challenge (–62.7 ± 8.1 pA/pF, n = 15, P < 0.001; Figure 4, a–c). The SB203580-activated current appeared identical to that activated by hypertonic exposure, with a linear I-V relationship, and reversal near 0 mV (Figure 4, a and d). Substitution of extracellular Na+ with K+ did not affect current amplitude (–69.4 ± 12.5 pA/pF, n = 4); nor did exposure to the Cl– channel inhibitor NPPB (–43.6 ± 10.8 pA/pF, n = 4, P > 0.05). However, partial substitution of bath Na+ with Tris+ significantly inhibited inward current amplitude (measured at –80 mV) and shifted the reversal potential in a negative direction (inward current, –340.1 ± 56.5 pA, and reversal potential, –25.3 ± 2.6 mV, at [Na+] = 40 mM, n = 5, P < 0.001; and inward current, –168.2 ± 14.8 pA, and reversal potential, –40.5 ± 4.2 mV at [Na+] = 20 mM, n = 4, P < 0.001) compared with control ([Na+] = 140 mM, –941.2 ± 84.5 pA inward current, and 0 ± 0.1 mV reverse potential, n = 9).
Figure 4.
Inhibition of p38 MAP kinase increases membrane Na+ permeability. Whole-cell currents were measured under basal conditions and during exposure to the p38 inhibitor SB203580 (500 nM). (a) Representative whole-cell recordings. Currents at –80 mV (downward deflection of the current trace) correspond to INa+. Under basal conditions, currents were small (first tracing). Exposure to SB203580 stimulated an increase in currents (second tracing) with properties similar to those activated by hypertonic exposure (20 mM sucrose, third tracing). Exposure to SB203580 did not further increase current magnitude after cells were first exposed to hypertonicity (50 mM sucrose, ∼350 mOsm) to maximally activate currents (fourth tracing). (b) Voltage-step protocol (as described in Figure 2a) demonstrating currents under control conditions and following exposure to SB203580 (500 nM). (c) Cumulative data recorded as average current density at –80 mV. Both SB203580 (500 nM) and hypertonicity (50 mM sucrose) resulted in large increases in inward currents. The responses were not additive. (d) I-V relationship of whole-cell currents measured under basal conditions and during exposure to SB203580. With Na+ as the primary extracellular cation, currents were characterized by a nearly linear I-V relationship and reversal near 0 mV. Partial replacement of Na+ with Tris+ (final [Na+] = 20 mM) decreased inward currents and shifted the reversal potential to –40.5 ± 4.2 mV, as expected for a cation-selective conductance.
The SB203580-activated currents also exhibited similar regulatory properties, in that (a) inclusion of EGTA (5 mmol/l, no added Ca2+) in the patch pipette abolished the response (–8.5 ± 2.1 pA/pF, n = 5, P < 0.01; Figure 5, open bars), and (b) exposure to amiloride (100 μM) inhibited currents by approximately 61% (–38.36 ± 10.8 pA/pF, n = 4) compared with control cells (–99.54 ± 6.55 pA/pF, n = 10, P < 0.001; Figure 5, open bars). Lastly, the hypertonic-induced and SB203580-activated currents were not additive. When currents were first activated by hypertonic exposure (50 mM sucrose, –69.5 ± 11.2 pA/pF, n = 6) there was no further increase caused by exposure to SB203580 (500 nM, –70.73 ± 7.65 pA/pF, n = 6; Figure 4, a and c). Taken together, these findings indicate that currents activated by SB203580 are not distinguishable from currents activated by hypertonic exposure, and suggest an important regulatory role for p38 MAP kinase in inhibition of volume-sensitive Na+ permeability.
Inhibition of p38 activity increases cell size.
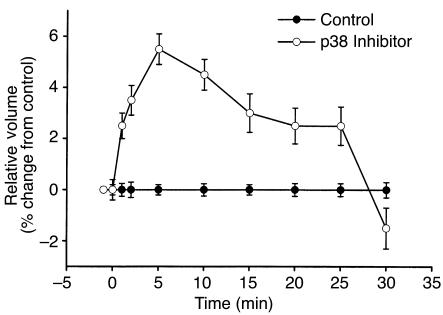
Since inhibition of p38 increases membrane Na+ permeability, the resulting influx of Na+ would be expected to increase cell volume. To assess this directly, cell volume was measured in the presence or absence of p38 inhibition (Figure 6). Compared with control, acute inhibition of p38 with SB203580 (500 nM) resulted in a rapid initial increase in cell size (5.8% ± 0.1%, n = 5, P < 0.01), reaching a maximum at 5 minutes. This was followed by a gradual return to basal values by 30 minutes. This gradual decrease in cell size likely represents an adaptive response to cell swelling, as previously described in these and other cell types (25, 34). Thus, constitutive activity of p38 serves an important role in the maintenance of cell volume and membrane Na+ permeability under isotonic conditions.
Figure 6.
Inhibition of p38 increases cell volume. Cell volume was measured in the presence or absence of p38 inhibitor using a Coulter Multisizer. Compared with control, acute inhibition of p38 with SB203580 (500 nM) resulted in a rapid initial increase in cell size (5.8 ± 0.1%, n = 5, P < 0.01), reaching a maximum at 5 minutes. This was followed by a gradual return to basal values by 30 minutes.
Intracellular dialysis with purified p38 MAP kinase inhibits channel opening.
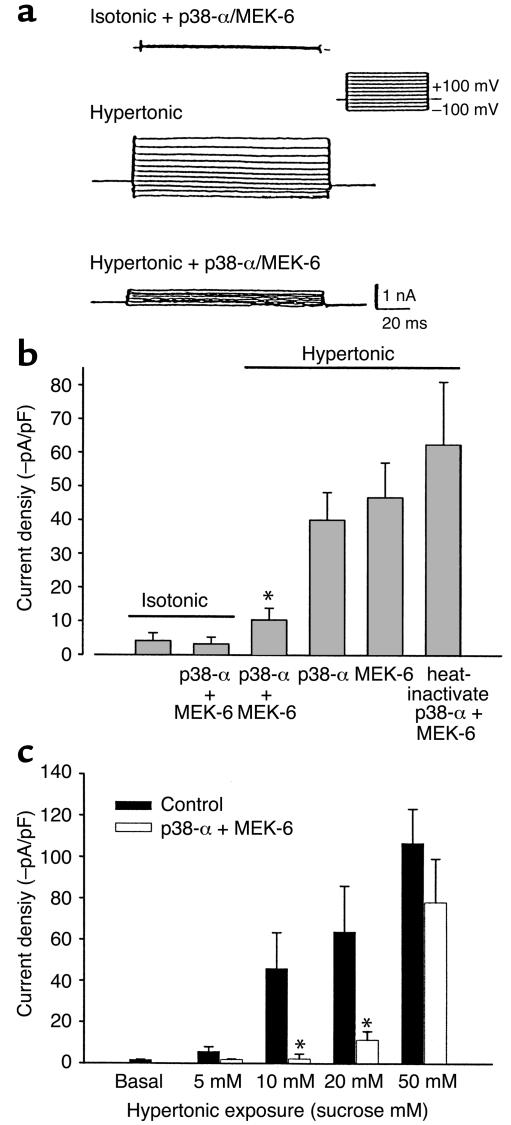
Since kinase inhibition can have unanticipated effects on other regulatory pathways, an alternate strategy was used to assess the relationship between p38 MAP kinase and Na+ permeability. p38α is activated by dual phosphorylation of adjacent tyrosine and threonine residues by the upstream MAP kinase kinase, MEK-6 (35–37). To determine if activated p38α MAP kinase inhibits channel function, the purified kinases p38α (5 μg/ml) and MEK-6 (5 μg/ml) were delivered, individually or together, to the cell interior by inclusion in the patch pipette. There was no effect by p38α and MEK-6 together on basal currents measured under isotonic conditions (Figure 7, a and b). In control cells dialyzed with heat-inactivated (100°C, 30 minutes) p38α and MEK-6, hypertonic exposure (20 mM sucrose, ∼320 mOsm; Figure 7) resulted in characteristic current activation (–62.5 ± 18.7 pA/pF, n = 5). Dialysis with either p38α or MEK-6 individually resulted in partial inhibition (–40.1±8.2 pA/pF and –46.8±10.3 pA/pF, n = 4, respectively; Figure 7, values not statistically significant compared with control). However, dialysis with p38α and MEK-6 together resulted in significant inhibition of the response to hypertonic exposure (–10.4 ± 3.5 pA/pF, n = 6, P < 0.01; Figure 7, a and b). To assess whether the inhibitory effects of recombinant p38α + MEK-6 were detected over a broader range of osmotic challenge, cells were exposed to graded increases in osmolarity (Figure 7c). Under control conditions, the current amplitude increased with increasing transmembrane osmolar gradients. In the presence of intracellular p38α + MEK-6, there was significant inhibition of current amplitude at lower transmembrane osmolar gradients (10 mM and 20 mM sucrose, P < 0.01). The inhibitory effect of p38α + MEK-6 was overcome at higher transmembrane osmolar gradients (50 mM sucrose, P > 0.05 compared with control at 50 mM sucrose).
Figure 7.
Intracellular dialysis with recombinant p38 MAP kinase protein inhibits volume-sensitive current activation. Under whole-cell patch-clamp conditions, the recombinant kinases p38α (5 μg/ml) and MEK-6 (5 μg/ml) were delivered, individually or together, to the cell interior by inclusion in the patch pipette, and cells were then exposed to hypertonic buffer (20 mM sucrose, ∼320 mOsm). (a) Whole-cell currents measured using the voltage-step protocol (as described in Figure 2a). Currents were small with intracellular dialysis of p38α and MEK-6 under isotonic conditions (top tracing). However, p38α and MEK-6 significantly inhibit the amplitude of hypertonic-induced currents (bottom tracing) as compared with control (middle tracing). (b) Cumulative data expressed as average current density at –80 mV. Control cells, dialyzed with heat-inactivated p38α and MEK-6, demonstrated characteristic current activation. Dialysis with either p38α or MEK-6 individually resulted in partial inhibition, which was not statistically significant compared with control. However, dialysis with p38α and MEK-6 together resulted in significant current inhibition. There was no effect of p38α and MEK-6 on basal (isotonic) currents. (c) Under control conditions, current density increased with increasing transmembrane osmolar gradients. In the presence of intracellular p38α + MEK-6, there was significant inhibition of current amplitude at lower transmembrane osmolar gradients (5–20 mM sucrose). However, this inhibitory effect was overcome at higher transmembrane osmolar gradients (50 mM sucrose). *P < 0.01.
Discussion
Environmental signals such as ultraviolet radiation, heat shock, and osmotic stress cause p38 MAP kinase activation in several cell types (4, 7, 8). While many of the physiologic effects of p38 are mediated by transcriptional regulation, there is increasing evidence that p38 can interact with and modulate other cytoplasmic and membrane proteins in a phosphorylation-dependent manner. In these studies of HTC cells, observations using a variety of techniques support a broader role for p38 MAP kinase as an early and important signal in coordinating changes in cell volume and membrane Na+ permeability.
The principal findings of these studies are that (a) there is constitutive activity of p38 MAP kinase under basal (isotonic) conditions; (b) inhibition of constitutive p38 MAP kinase results in increased membrane Na+ permeability and increases in cell volume; (c) intracellular dialysis with purified p38α, and its upstream activator MEK-6, inhibits volume-sensitive channel opening; and (d) exposure to hypotonicity to increase cell volume results in large increases in p38 activity. Collectively, these findings suggest that p38 MAP kinase plays an important regulatory role governing membrane Na+ permeability and cell volume regulation through inhibitory effects on NSC channels.
Previous biophysical studies have identified NSC channels in liver cells that are regulated in part by cytosolic [Ca2+] (18, 20). While channel proteins are abundant, with approximately 2,000 channels/cell, they are generally closed under basal conditions. Channels open in response to vasopressin or other agents known to mobilize Ca2+ (23), and in response to cell shrinkage induced by osmotic (17) or oxidative stress (20). The resulting influx of Na+ favors water movement into the cell and restoration of cell volume toward basal values. Interestingly, p38 MAP kinase is known to be constitutively active in hepatocytes, and its activity is selectively decreased during oxidative stress (38), consistent with a potential role as an inhibitor of Na+ influx.
These studies provide further evidence that NSC channels are crucial to the maintenance of cell volume and RVI in hepatocytes. The conductance is characterized by equal permeability for Na+ and K+ and a linear I-V relationship, and shows no time dependence. Partial substitution of extracellular Na+ with Tris+ causes a decrease in inward currents and a negative shift in reversal potential, as anticipated for an NSC conductance. It should be noted, however, that a slight decrease in the amplitude of outward currents was also observed with Tris+ substitution. The possibility that Tris+ is also a partial channel blocker, or has other nonspecific effects on cell volume, intracellular pH, or other parameters that may affect channel open probability cannot be excluded.
The partial inhibition of volume-sensitive conductance by amiloride is consistent with previous studies of rat hepatocytes (16). Previous reports in other cell types have demonstrated variable effects of amiloride on NSC currents (39–42). This variability may reflect different channel types; the molecular identity of NSC channels in liver cells has not been defined. The basic biophysical properties of the NSC channels described here appear similar to cyclic nucleotide–gated channels described in other cell types (43, 44) that demonstrate amiloride sensitivity (45–47). The finding that amiloride only partially inhibits NSC conductance has several potential explanations. First, amiloride sensitivity may be modulated by [Ca2+] (39, 48), mechanical stress (49), or other factors. Alternatively, more than one Na+ channel type may contribute to volume-regulated Na+ influx. In fact, the epithelial Na+ channel (ENaC), which is also inhibited by amiloride, has recently been implicated in volume-stimulated Na+ influx in rat hepatocytes (33). However, the NSC conductance of HTC cells does not exhibit the pore or regulatory properties anticipated for ENaC. Thus, it will be important to define the molecular mechanisms responsible for volume-sensitive Na+ influx, and assess the specific regulatory pathways involved for each.
Two primary observations support a role for constitutive p38 MAP kinase activity in the regulation of cell volume. First, under isotonic conditions, inhibition of p38 with SB203580 increased membrane Na+ permeability. Currents activated by SB203580 demonstrated biophysical properties that were identical to those of currents activated by hypertonic exposure, including a linear I-V relationship, equal permeability to Na+ and K+, Ca2+-dependent regulation, and amiloride sensitivity. Second, exposure to SB203580 was followed by an increase in cell volume (∼6%), consistent with an important role for p38 MAP kinase in the maintenance of resting cell volume under isotonic conditions.
While the inhibitory effects of SB203580 are detectable at low micromolar concentrations and appear to be specific for p38 (50, 51), it is acknowledged that SB203580 could have unanticipated effects on other signaling pathways. Consequently, an alternative strategy was used to evaluate the roles of recombinant p38α and the upstream MAP kinase kinase MEK-6 on channel regulation. Intracellular delivery of these kinases inhibited volume-sensitive current activation. Indeed, intracellular delivery of active p38α resulted in a large shift in the activation curve, so that much higher degrees of hypertonicity were required before channel opening was observed. Dialysis with either p38α or MEK-6 alone resulted in only partial inhibition of currents, and dialysis with heat-inactivated p38α had no effect. Thus, p38α, when present with its specific activator, is capable of channel inhibition. The partial effect observed with either p38α or MEK-6 alone may be related to activation of these proteins by endogenous cellular kinases. It is important to note that at higher degrees of hypertonicity (50 mM sucrose), NSC conductance could be activated even in the presence of p38α and MEK-6. Thus, the inhibitory effect of p38 MAP kinase can be overcome by positive regulatory signals. In fact, there was no observable decrease in p38 activity with hypertonic exposure. Consequently, it will be important to define the additional regulatory pathways (regulatory proteins, cytoskeletal elements, insertion of new channels into the membrane, etc.) that are able to overcome the inhibitory effects of p38 and stimulate channel opening during hypertonic conditions.
The effects of p38 MAP kinase on membrane Na+ permeability have several implications. First, while p38 has previously been shown to be an important regulator of transcription, these effects on Na+ permeability imply that p38 MAP kinase has plasma membrane targets as well. Indeed, recent evidence suggests that p38 may modulate the Na+/H+ exchanger (NHE-1) in vascular smooth muscle cells (15), Ca2+-activated, voltage-gated channels in neuronal cells (14), and acid secretion from gastric parietal cells (52). In all cases, p38 appears to have an inhibitory role, as observed here. However, whether p38 regulates channel function through a direct phosphorylation event or through downstream kinase pathways is yet to be determined. Second, while MAP kinase pathways were thought to mediate the effects of growth factors and hormones on sustained cellular events such as proliferation and differentiation, recent evidence has now emerged that MAP kinase pathways can also be activated by heterotrimeric G proteins (53, 54) for rapid regulation of effector pathways (55, 56). For example, p38β appears to be involved in the regulation of N-type calcium currents by bradykinin in a neuronal cell line, a response that occurs within seconds (14). Additionally, in neutrophils, p38 is activated within 2 minutes of exposure to formyl-methionyl-leucyl-phenylalanine (fMLP), an inflammatory stimulus, and modulates the response to hypertonic exposure (57). These studies support roles for p38 MAP kinase in rapid regulation of cellular events that are not necessarily related to gene transcription.
Assuming that p38 functions as a primary signal governing Na+ influx and resting cell volume, several additional points merit further investigation. First, because the molecular identity of the NSC channel has not been established, the cellular site(s) of action of p38 is not clear. p38 may modulate channel activity by direct phosphorylation, or conversely, through effects on downstream kinases or phosphatases. Second, functional interactions between p38 and other kinases are likely to be operative. For example, preliminary evidence suggests that tyrosine kinase activity is also important in the response to cell volume changes (58, 59), and Src tyrosine kinase has been shown to be a direct regulator of ion channel function in different cell types (60). The sequence of action and relative importance of p38 versus other kinases has not been established, and is likely to be cell-type specific. For example, it should be noted that while PD98059 did not inhibit the response to hypertonic exposure, a role of ERK in channel regulation through MEK-1–independent pathways cannot be excluded. Lastly, a broad range of physiologic and pathologic stimuli modulate NSC channel activity, though the role of p38 in the mediation of these responses is largely unknown. However, p38 has been shown to play an important role in the insulin signaling pathway (61) as well as in responses to oxidative stress (38) and the initiation of apoptosis (62, 63), processes that are associated with alterations in cell volume (20, 64).
Taken together, these findings indicate that in HTC cells, p38 MAP kinase plays a key role in tonic inhibition of Na+ permeability and maintenance of cell volume. It is clear, however, that the inhibitory effects of p38 MAP kinase are opposed by intracellular [Ca2+] and presumably other stimulatory signals that work in concert to modulate Na+ permeability in response to changing physiologic demands. Definition of the complex signaling pathways involved may provide new strategies for modulating liver cell function through effects on cell volume, and for minimizing cell injury caused by sustained Na+ influx.
Acknowledgments
This study was supported by the Children’s Hospital Research Institute Professional Development Award, the American Gastroenterological Association, the American Digestive Health Foundation, the Children’s Digestive Health and Nutrition Foundation (A.P. Feranchak), and National Institute of Diabetes, Digestive and Kidney Diseases grants DK-43278 and DK-46082 (J.G. Fitz).
References
- 1.Paul A, et al. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 2.Bode JG, et al. The mitogen-activated protein (MAP) kinase p38 and its upstream activator MAP kinase kinase 6 are involved in the activation of signal transducer and activator of transcription by hyperosmolarity. J Biol Chem. 1999;274:30222–30227. doi: 10.1074/jbc.274.42.30222. [DOI] [PubMed] [Google Scholar]
- 3.Han J, Richter B, Li Z, Kravchenko V, Ulevitch RJ. Molecular cloning of human p38 MAP kinase. . Biochim Biophys Acta. 1995; 1265:224–227. doi: 10.1016/0167-4889(95)00002-a. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 5.Derijard B, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms [erratum 1995, 269(5220):17] Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 6.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raingeaud J, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 8.Kyriakis JM, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 9.Teague TK, et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci USA. 1999;96:12691–12696. doi: 10.1073/pnas.96.22.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadkarni V, Gabbay KH, Bohren KM, Sheikh-Hamad D. Osmotic response element enhancer activity. Regulation through p38 kinase and mitogen-activated extracellular signal-regulated kinase kinase. J Biol Chem. 1999;274:20185–20190. doi: 10.1074/jbc.274.29.20185. [DOI] [PubMed] [Google Scholar]
- 11.Wondergem R, Davis J. Ethanol increases hepatocyte water volume. Alcohol Clin Exp Res. 1994;18:1230–1236. doi: 10.1111/j.1530-0277.1994.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 12.Serrar H, Haddad P. Effects of cold preservation and rewarming on rat liver cell volume regulation and concentrative amino acid uptake. Gastroenterology. 1997;112:1344–1353. doi: 10.1016/s0016-5085(97)70148-3. [DOI] [PubMed] [Google Scholar]
- 13.Wettstein M, Haussinger D. Cytoprotection by the osmolytes betaine and taurine in ischemia-reoxygenation injury in the perfused rat liver. Hepatology. 1997;26:1560–1566. doi: 10.1053/jhep.1997.v26.pm0009397998. [DOI] [PubMed] [Google Scholar]
- 14.Wilk-Blaszczak MA, et al. The mitogen-activated protein kinase p38-2 is necessary for the inhibition of N-type calcium current by bradykinin. J Neurosci. 1998;18:112–118. doi: 10.1523/JNEUROSCI.18-01-00112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusuhara M, et al. p38 Kinase is a negative regulator of angiotensin II signal transduction in vascular smooth muscle cells: effects on Na+/H+ exchange and ERK1/2. Circ Res. 1998;83:824–831. doi: 10.1161/01.res.83.8.824. [DOI] [PubMed] [Google Scholar]
- 16.Wehner F, Sauer H, Kinne RKH. Hypertonic stress increases the Na+ conductance of rat hepatocytes in primary culture. J Gen Physiol. 1995;105:507–535. doi: 10.1085/jgp.105.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehner F, Tinel H. Role of Na+ conductance, Na+-H+ exchange and Na+-K+-2Cl- symport in the regulatory volume increase of rat hepatocytes. J Physiol. 1998;506:127–142. doi: 10.1111/j.1469-7793.1998.127bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidofsky SD, Sostman A, Fitz JG. Regulation of cation-selective channels in liver cells. J Membr Biol. 1997;157:231–236. doi: 10.1007/s002329900231. [DOI] [PubMed] [Google Scholar]
- 19.Fitz JG, Sostman A, Middleton JP. Regulation of cation channels in liver cells by intracellular calcium and protein kinase C. Am J Physiol. 1994;266:G677–G684. doi: 10.1152/ajpgi.1994.266.4.G677. [DOI] [PubMed] [Google Scholar]
- 20.Schlenker T, Feranchak AP, Schwake L, Stremmel W, Fitz JG. Functional interactions between oxidative stress, membrane Na+ permeability, and cell volume in hepatoma cells. Gastroenterology. 2000;118:395–403. doi: 10.1016/s0016-5085(00)70222-8. [DOI] [PubMed] [Google Scholar]
- 21.Bodily K, Wang Y, Roman RM, Sostman A, Fitz JG. Characterization of a swelling-activated anion conductance in HTC hepatoma cells. Hepatology. 1997;25:403–410. doi: 10.1002/hep.510250224. [DOI] [PubMed] [Google Scholar]
- 22.Chang D, Hsieh PS, Dawson DC. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med. 1988;18:351–366. doi: 10.1016/0010-4825(88)90022-4. [DOI] [PubMed] [Google Scholar]
- 23.Lidofsky SD, Xie MH, Sostman A, Scharschmidt BF, Fitz JG. Vasopressin increases cytosolic sodium concentration in hepatocytes and activates calcium influx through cation-selective channels. J Biol Chem. 1993;268:14632–14636. [PubMed] [Google Scholar]
- 24.Fitz JG, Sostman A. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. Am J Physiol. 1994;266:G544–G553. doi: 10.1152/ajpgi.1994.266.4.G544. [DOI] [PubMed] [Google Scholar]
- 25.Roman RM, et al. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- 26.Berl T, Siriwardana G, Ao L, Butterfield LM, Heasley LE. Multiple mitogen-activated protein kinases are regulated by hyperosmolality in mouse IMCD cells. Am J Physiol. 1997;272:F305–F311. doi: 10.1152/ajprenal.1997.272.3.F305. [DOI] [PubMed] [Google Scholar]
- 27.Wojtaszek PA, Heasley LE, Siriwardana G, Berl T. Dominant-negative c-Jun NH2-terminal kinase 2 sensitizes renal inner medullary collecting duct cells to hypertonicity-induced lethality independent of organic osmolyte transport. J Biol Chem. 1998;273:800–804. doi: 10.1074/jbc.273.2.800. [DOI] [PubMed] [Google Scholar]
- 28.Sluss HK, Barrett T, Derijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young PR, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors—mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 32.New L, et al. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohmer C, Wehner F. The epithelial Na(+) channel (ENaC) is related to the hypertonicity-induced Na(+) conductance in rat hepatocytes. FEBS Lett. 2001;494:125–128. doi: 10.1016/s0014-5793(01)02303-1. [DOI] [PubMed] [Google Scholar]
- 34.Feranchak AP, Roman RM, Schwiebert EM, Fitz JG. Phosphatidylinositol 3-kinase represents a novel signal regulating cell volume through effects on ATP release. J Biol Chem. 1998;273:14906–14911. doi: 10.1074/jbc.273.24.14906. [DOI] [PubMed] [Google Scholar]
- 35.Han J, et al. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 36.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 37.Stein B, Brady H, Yang MX, Young DB, Barbosa MS. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- 38.Mendelson KG, Contois L-R, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci USA. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tohda H, Foskett JK, O’Brodovich H, Marunaka Y. Cl- regulation of a calcium-activated nonselective cation channel in beta-agonist-treated fetal distal lung epithelium. Am J Physiol. 1994;266:C104–C109. doi: 10.1152/ajpcell.1994.266.1.C104. [DOI] [PubMed] [Google Scholar]
- 40.Koch JP, Korbmacher C. Mechanism of shrinkage activation of nonselective cation channels in M-1 mouse cortical collecting duct cells. J Membr Biol. 2000;177:231–242. doi: 10.1007/s002320010006. [DOI] [PubMed] [Google Scholar]
- 41.Marunaka Y, Niisato N. The essential role of cytosolic Cl- in Ca2+ regulation of an amiloride-sensitive channel in fetal rat pneumocyte. J Membr Biol. 2001;180:91–99. doi: 10.1007/s002320010061. [DOI] [PubMed] [Google Scholar]
- 42.Chiba T, Marcus DC. Nonselective cation and BK channels in apical membrane of outer sulcus epithelial cells. J Membr Biol. 2000;174:167–179. doi: 10.1007/s002320001041. [DOI] [PubMed] [Google Scholar]
- 43.Broillet MC, Firestein S. Cyclic nucleotide-gated channels. Molecular mechanisms of activation. Ann NY Acad Sci. 1999;868:730–740. doi: 10.1111/j.1749-6632.1999.tb11352.x. [DOI] [PubMed] [Google Scholar]
- 44.Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 45.Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol. 1992;100:45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandorpe DH, Ciampolillo F, Green RB, Stanton BA. Cyclic nucleotide-gated cation channels mediate sodium absorption by IMCD (mIMCD-K2) cells. Am J Physiol. 1997;272:C901–C910. doi: 10.1152/ajpcell.1997.272.3.C901. [DOI] [PubMed] [Google Scholar]
- 47.Kleene SJ. Spontaneous gating of olfactory cyclic-nucleotide-gated channels. J Membr Biol. 2000;178:49–54. doi: 10.1007/s002320010014. [DOI] [PubMed] [Google Scholar]
- 48.Marunaka Y, Tohda H, Hagiwara N, O’Brodovich H. Cytosolic Ca(2+)-induced modulation of ion selectivity and amiloride sensitivity of a cation channel and beta agonist action in fetal lung epithelium. Biochem Biophys Res Commun. 1992;187:648–656. doi: 10.1016/0006-291x(92)91244-k. [DOI] [PubMed] [Google Scholar]
- 49.Marunaka Y, Tohda H, Hagiwara N, Nakahari T. Antidiuretic hormone-responding nonselective cation channel in distal nephron epithelium (A6) Am J Physiol. 1994;266:C1513–C1522. doi: 10.1152/ajpcell.1994.266.6.C1513. [DOI] [PubMed] [Google Scholar]
- 50.Whitmarsh AJ, Yang SH, Su MS, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, et al. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6:1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 52.Pausawasdi N, Ramamoorthy S, Stepan V, del Valle J, Todisco A. Regulation and function of p38 protein kinase in isolated canine gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G24–G31. doi: 10.1152/ajpgi.2000.278.1.G24. [DOI] [PubMed] [Google Scholar]
- 53.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi J, Nagao M, Kaziro Y, Itoh H. Activation of p38 mitogen-activated protein kinase by signaling through G protein-coupled receptors. Involvement of Gbetagamma and Galphaq/11 subunits. J Biol Chem. 1997;272:27771–27777. doi: 10.1074/jbc.272.44.27771. [DOI] [PubMed] [Google Scholar]
- 55.Ruis H, Schuller C. Stress signaling in yeast. Bioessays. 1995;17:959–965. doi: 10.1002/bies.950171109. [DOI] [PubMed] [Google Scholar]
- 56.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 57.Junger WG, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krump E, Nikitas K, Grinstein S. Induction of tyrosine phosphorylation and Na+/H+ exchanger activation during shrinkage of human neutrophils. J Biol Chem. 1997;272:17303–17311. doi: 10.1074/jbc.272.28.17303. [DOI] [PubMed] [Google Scholar]
- 59.Szaszi K, Buday L, Kapus A. Shrinkage-induced protein tyrosine phosphorylation in Chinese hamster ovary cells. J Biol Chem. 1997;272:16670–16678. doi: 10.1074/jbc.272.26.16670. [DOI] [PubMed] [Google Scholar]
- 60.Hilborn MD, Vaillancourt RR, Rane SG. Growth factor receptor tyrosine kinases acutely regulate neuronal sodium channels through the src signaling pathway. J Neurosci. 1998;18:590–600. doi: 10.1523/JNEUROSCI.18-02-00590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heidenreich KA, Kummer JL. Inhibition of p38 mitogen-activated protein kinase by insulin in cultured fetal neurons. J Biol Chem. 1996;271:9891–9894. doi: 10.1074/jbc.271.17.9891. [DOI] [PubMed] [Google Scholar]
- 62.Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 63.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 64.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]