Abstract
Infection with Helicobacter pylori causes chronic gastritis, which is characterized by a dense mucosal infiltration by inflammatory cells such as monocytes/macrophages. H. pylori–induced inflammation is a risk factor for the development of gastric adenocarcinoma, but the mechanisms involved in H. pylori–associated carcinogenesis are poorly understood. A cecropin-like H. pylori peptide, Hp(2-20), was found to be a monocyte chemoattractant and activated the monocyte NADPH-oxidase to produce oxygen radicals. The receptors mediating monocyte activation were identified as FPRL1 and the monocyte-specific orphan receptor FPRL2. Hp(2-20)–activated monocytes inhibited lymphocytes with antitumor properties, such as CD56+ natural killer (NK) cells and CD3ε+ T cells. The changes observed in NK cells and T cells — a reduced antitumor cytotoxicity, downregulation of CD3ζ expression, and apoptosis — were mediated by Hp(2-20)–induced oxygen radicals. Histamine, a gastric mucosal constituent, rescued NK cells and T cells from inhibition and apoptosis by suppressing Hp(2-20)–induced oxygen radical formation. We conclude that H. pylori expression of this monocyte-activating peptide contributes to its ability to attract and activate monocytes and reduces the function and viability of antineoplastic lymphocytes. These novel mechanisms may be subject to local, histaminergic regulation in the gastric mucosa.
Introduction
Compelling epidemiological evidence links the chronic gastritis associated with infection with Helicobacter pylori to the development of gastric adenocarcinoma (1), the second leading cause of cancer-related death in the world (2). One of several mechanisms that have been proposed to account for the increased cancer risk is that gastric carcinoma occurs as the result of an inappropriately regulated local host immune response to the infection. A general feature of this response is a dense infiltration of the subepithelial gastric lamina propria by phagocytes, mainly monocyte/macrophages and neutrophilic granulocytes, and lymphocytes, including those of relevance to defense against arising and established gastric cancer such as natural killer (NK) cells and T cells (3–6).
To further understand the mechanisms underlying H. pylori–induced carcinogenesis, it is of interest to explore interactions between H. pylori and leukocytes infiltrating the infected tissue. H. pylori cause a chronic, often lifelong infection of the gastric epithelial cells, but the bacteria do not normally penetrate into the subepithelial lamina propria. Thus, modulation of leukocyte function is likely to depend on the release of soluble bacterial products (7–9). In this study, we have investigated the immunomodulatory properties of a cecropin-like H. pylori–derived peptide, Hp(2–20) (10, 11). We show that Hp(2–20), acting via two receptors of the FPR family of chemoattractant receptors, attracts monocytes and activates monocytes to generate NADPH-oxidase–derived oxygen radicals. Monocytes activated by Hp(2–20) suppressed functions of NK cells and T cells and triggered apoptosis in both cell types; these inhibitory events were mediated by oxygen radicals. Histamine, a gastric mucosal constituent, protected NK cells/T cells from monocyte-induced functional inhibition and apoptosis. We hypothesize that Hp(2–20) contributes to the accumulation and activation of monocytes in chronic gastritis, and that the Hp(2–20)–induced formation of mutagenic oxygen radicals with ensuing inhibition of antineoplastic lymphocytes may be of relevance to the increased cancer risk in H. pylori–infected gastric tissue.
Methods
Peptides and chemicals.
The peptides used, Hp(2–20), AKKVFKRLEKLFSKIQNDK, and WKYMVm, were synthesized and handled as described (11). Histamine dihydrochloride was from Maxim Pharmaceuticals (San Diego, California, USA), ranitidine hydrochloride from Glaxo (Mölndal, Sweden), and human recombinant IL-2 from Genzyme (Stockholm, Sweden).
Separation of leukocytes.
Peripheral blood was obtained from blood donors at Sahlgren’s Hospital, Göteborg, Sweden. After Ficoll-Hypaque centrifugation (12), mononuclear cells were separated into lymphocytes and monocytes using the countercurrent centrifugal elutriation (CCE) technique, as described in detail elsewhere (12). This procedure yielded one fraction with >90% monocytes (at a flow rate of 20–22 ml/min) and two lymphocyte fractions, one enriched for CD3ε–/56+ NK cells (45–50%, at 15–16 ml/min) and one enriched for CD3ε+/56– T cells (70–80%, at 13–14 ml/min).
Monocyte chemotaxis and NADPH-oxidase activity.
Monocyte chemotaxis was determined using ChemoTx multiwell chambers (Neuro Probe Inc., Gaithersburg, Maryland, USA) according to instructions provided by the manufacturer. Monocytes were allowed to migrate through the filters, and accumulation of cells in the lower compartments was determined microscopically after a 90-minute incubation at 37°C. NADPH-oxidase activity was determined using an isoluminol-enhanced chemiluminescence (CL) system that quantitates extracellular reactive oxygen species (ROS) (13).
Assays of apoptosis.
Apoptosis was monitored by use of flow cytometry, as described elsewhere (14). T cells or NK cells were gated after exposure to monocytes, and the gate was set to comprise lymphocytes with a reduced forward scatter and an increased right-angle scatter characteristic of apoptosis (12). Two additional methods were used to determine apoptosis in NK cells and T cells: analysis of DNA strand breaks by TUNEL assay and annexin V staining, as described elsewhere (14, 15).
Detection of lymphocyte surface and intracellular antigens.
One million cells were stained with appropriate FITC- and phycoerythrin-conjugated (PE-conjugated) mAb’s (Becton Dickinson, Stockholm, Sweden; 10 μl/106 cells), as described elsewhere (15). Cells were analyzed by use of flow cytometry on a FACSort with a Lysys II software program (Becton Dickinson). Lymphocytes were gated on the basis of forward and right-angle scatter. The flow rate was adjusted to less than 200 cells × s–1, and at least 5 × 103 cells were analyzed for each sample.
Lymphocytes analyzed for CD3ζ expression were first stained for appropriate surface antigens. Thereafter, cells were fixed and permeabilized using a Cytofix/Cytoperm Kit (Becton Dickinson) and incubated with PE-conjugated mAb’s against CD3ζ (TcRζ/TIA-2; Immunotech/Coulter, Marseille, France) according to the protocol provided by the manufacturer. Finally, the cells were analyzed for CD3ζ expression by use of flow cytometry.
Assay of NK cell cytotoxicity.
NK cell–mediated cytotoxicity against K562, an NK cell–sensitive leukemic cell line, was assayed as described elsewhere (14). NK cell–enriched lymphocytes (100,000 cells/well) were incubated in quadruplicates in 96-well microplates (Nunc A/S, Roskilde, Denmark) in the presence or absence of autologous monocytes (10,000–100,000 cells/well). All compounds were added at the onset of incubation, with the exception of formylmethionyl-leucyl-phenylalanine (fMLF; 0.1 μM) or Hp(2–20) (50 μM), which were added 20 minutes later. Finally, 10,000 51Cr-loaded (Amersham, Stockholm, Sweden) K562 cells were added to the cell suspension. After incubation at 37°C for 16 hours, supernatant fluids were collected by a tissue collecting system (Amersham) and assayed for radioactivity in a gamma-counter. NK cell cytotoxicity was calculated using the formula:
Equation 1
| 1 |
Cytosolic [Ca2+] in HL-60 cells expressing FPRL1, FPRL2, and FPR.
Stable expression of FPR, FPRL1, and FPRL2 in undifferentiated HL-60 cells was obtained as described, and their interaction with Hp(2–20) was determined by the ability of the peptide to mobilize intracellular [Ca2+] in fura 2–loaded cells (16).
Results
A cecropin-like H. pylori peptide activates human monocytes
Chemotaxis and NADPH-oxidase activity.
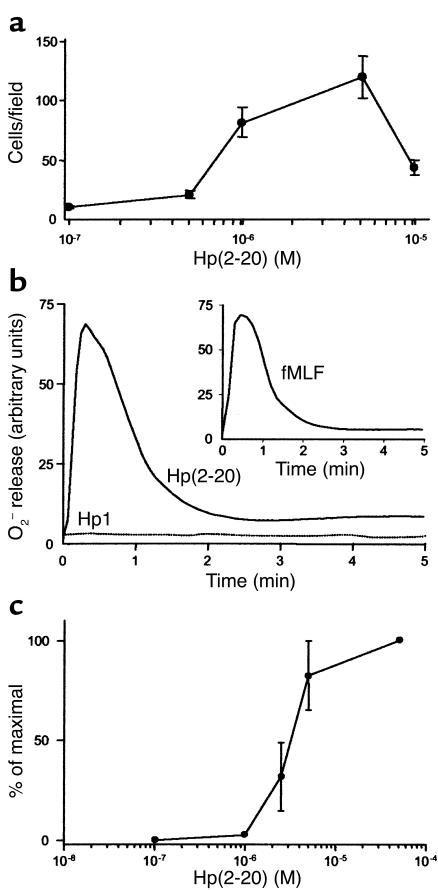
The Hp(2–20) peptide was found to be dose-dependently chemotactic for monocytes (Figure 1a). The maximal transmigration observed was only slightly lower than that induced by the well-characterized and efficacious monocyte chemoattractant fMLF. Monocyte chemoattractants commonly activate a specialized electron-transporting system, the NADPH-oxidase, which ferries electrons to molecular oxygen. In this way, oxygen is reduced to superoxide anions, which in turn is converted to toxic oxygen products (17). The Hp(2–20) peptide induced a robust and dose-dependent oxygen radical production (superoxide anion) in monocytes (Figure 1, b and c). The time course and magnitude of the response were comparable to those induced by fMLF (Figure 1b, inset). The Hp(2–20)–induced superoxide anion formation was inhibited by the NADPH-oxidase inhibitor DPI (18). A greater than 90% inhibition of Hp(2–20)–induced activity was observed at a DPI concentration of 10 μM. In parallel experiments, DPI inhibited oxygen radical formation in response to fMLF, an established NADPH-oxidase activator (17) with similar efficacy and potency (not shown). These results suggest that Hp(2–20) is a true activator of the monocyte NADPH-oxidase.
Figure 1.
Hp(2–20)–induced monocyte chemotaxis and activation of the monocyte NADPH-oxidase. Monocyte transmigration after 90 minutes in response to different Hp(2–20) concentrations was determined using a ChemoTx multiwell chamber system. Migration was determined microscopically by counting cells in the lower compartments. (a) Hp(2–20) induced chemotaxis in human monocytes in a dose-dependent manner. Mean values ± SEM of three separate experiments. Hp(2–20) (50 μM), but not the control peptide Hp1 (50 μM), triggered superoxide anion production in monocytes (b) with kinetics similar to that induced by the formylated peptide fMLF (inset). The Hp(2–20)–induced superoxide anion release was dose-dependent within the micromolar range (c). One representative experiment out of five (b and inset), and mean values ± SEM of five separate experiments (c).
An intact α-helical structure of Hp(2–20) is required for monocyte activation.
Hp(2–20) contains a perfect amphipathic α-helical structure, similar to those found in cecropins, which can be interrupted by replacing key amino acids (16, 19). Replacement of the lysine in position 9 of Hp(2–20) with leucine, an amino acid that lacks a polar side chain, interferes with the helical structure (19). No monocyte activation was obtained with the K→L substituted control peptide Hp1 (Figure 1b).
Hp(2–20) activates monocytes via FPRL1 and its monocyte-specific homologue FPRL2.
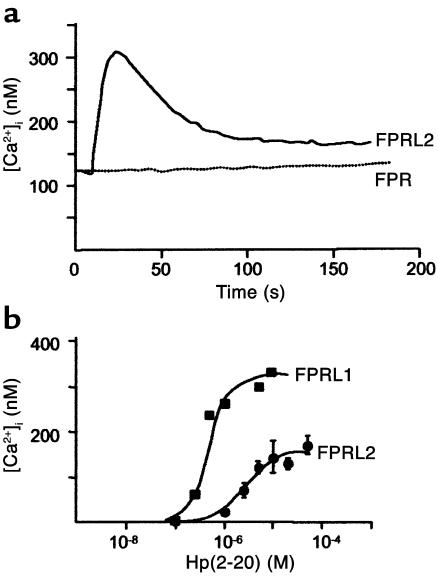
By using undifferentiated HL-60 cells that had been stably transfected with FPR, FPRL1, or FPRL2, it was found that Hp(2–20) activated monocytes via FPRL1 and FPRL2, but not via FPR. Thus, cells expressing the specific monocyte receptor FPRL2 responded with a rise in intracellular [Ca2+] reaching a level of approximately 300 nM (Figure 2a). The EC50 value of the Hp(2–20)–induced calcium mobilization in FPRL2-expressing cells was approximately 10 μM, whereas that for FPRL1 was approximately 30-fold lower (Figure 2b).
Figure 2.
Hp(2–20)–induced calcium mobilization in transfected HL-60 cells. Receptor interaction with Hp(2–20) (50 μM) was determined by measuring the ability of the peptide to mobilize intracellular calcium in undifferentiated HL-60 cells, stably transfected with either FPR, FPRL1, or FPRL2. Cells expressing the specific monocyte receptor FPRL2 responded with a rise in intracellular [Ca2+], which peaked at approximately 300 nM (a). The EC50 of the Hp(2–20)–induced calcium mobilization in FPRL2-expressing cells was around 10 μM, whereas that for FPRL1 was approximately 30-fold lower (b). No significant rise in intracellular [Ca2+] occurred when Hp(2–20) was added to cells expressing FPR (a).
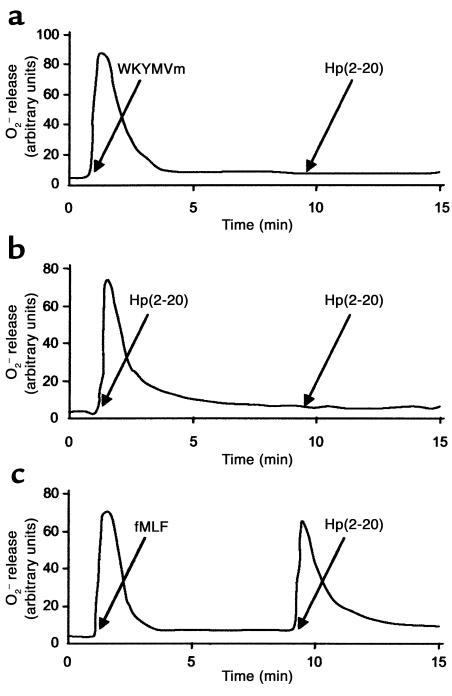
The supposition that Hp(2–20) activated monocytes via FPRL1 and FPRL2, but not via FPR, was confirmed in desensitization experiments using the agonists WKYMVm, which desensitizes FPR, FPRL1 and FPRL2, and fMLF, which desensitizes only FPR (20). Thus, monocytes first activated with WKYMVm were unable to generate a second burst of superoxide when subsequently challenged with Hp(2–20) (Figure 3a). A similar desensitization was observed after a reciprocal stimulation (not shown). Homologous desensitization with two subsequent stimulations of Hp(2–20) was also apparent (Figure 3b). No desensitization against Hp(2–20)–induced activation occurred in monocytes first challenged with fMLF (Figure 3c).
Figure 3.
Desensitization of the Hp(2–20) (50 μM) response. Monocytes first activated with the agonist WKYMVm (0.1 μM) (which desensitizes FPR, FPRL1 and FPRL2) were unable to generate a second burst of superoxide when challenged 10 minutes later with Hp(2–20) (a). Homologous desensitization with two subsequent stimulations of Hp(2–20) was also apparent (b). No desensitization against Hp(2–20)–induced activation was obtained in monocytes first challenged with fMLF (0.1 μM) (which desensitizes only FPR) (c).
Lymphocyte inhibition and apoptosis induced by Hp(2–20)
Earlier studies reveal that monocytes/macrophages trigger functional inhibition of NK cells (12, 15). Our finding that Hp(2–20) potently triggered superoxide anion formation in monocytes prompted us to investigate effects of Hp(2–20) on monocyte-NK cell interactions. For this purpose, we incubated monocytes at various densities with autologous NK cell–enriched lymphocytes and monitored NK cell function and viability.
NK cell cytotoxicity.
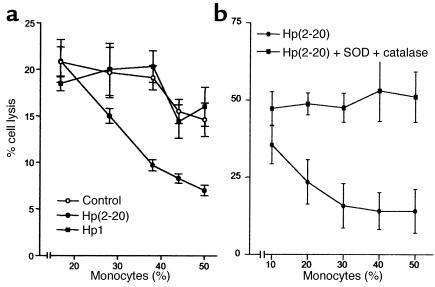
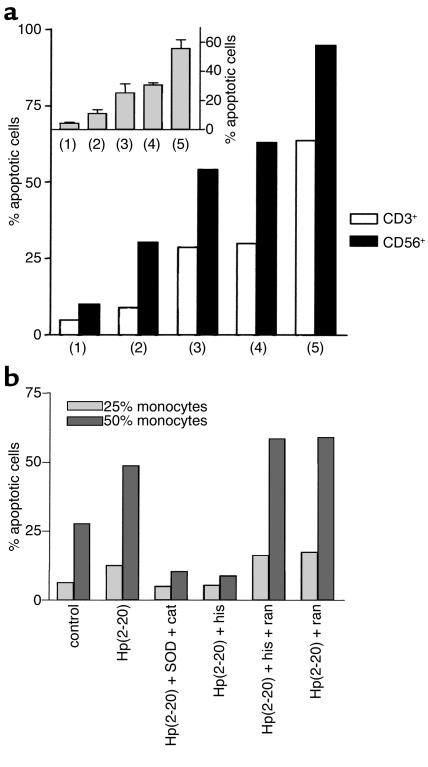
Hp(2–20) (50 μM), but not Hp1, effectively enhanced the monocyte-induced NK cell suppression (Figure 4a). The Hp(2–20)–induced inhibition was significantly more pronounced than that induced by control monocytes and completely counteracted by treatment with a combination of the oxygen radical scavengers SOD and catalase (Figure 4b). More than 90% of the lymphocyte cytotoxicity was depleted by the removal of CD56+ NK cells by use of anti–CD56-coated beads (14); in contrast, removal of CD3+ T cells by use of anti–CD3-coated beads did not significantly reduce cytotoxicity (not shown).
Figure 4.
Monocyte-dependent inhibition of NK cell cytotoxicity by Hp(2–20) (50 μM), reversal by oxygen radical scavengers. NK cell–enriched lymphocytes (105 cells/well) were incubated with autologous monocytes for 16 hours at 37°C and assayed for cytotoxicity against 51Cr-labeled K562 cells (104 cells/well). (a) The cell cultures were treated with culture medium (control, open circles), Hp(2–20) (filled circles), or the control peptide Hp1 (50 μM) (filled boxes). Results are percentage of cell lysis (mean ± SEM of quadruplicates). Hp(2–20) did not significantly affect cytotoxicity in the absence of monocytes (not shown) but enhanced the monocyte-induced inhibition of cytotoxicity at all monocyte/lymphocyte ratios investigated (a). The monocyte-dependent inhibition of NK cell cytotoxicity by Hp(2–20) was confirmed in ten experiments using blood from different donors. In these experiments, the Hp(2–20)–induced inhibition was statistically significant over that induced by control monocytes at 37% monocytes (P < 0.005; Wilcoxon’s rank sum test) and 44% monocytes (P < 0.005). (b) The cell cultures were treated with Hp(2–20) (filled circles) or Hp(2–20) + SOD (50 U/ml) + catalase (200 U/ml) (filled boxes). Results are percentage of cell lysis (mean ± SEM results from eight experiments using blood from different donors). At all monocyte/lymphocyte ratios above 1:5 monocytes, SOD + catalase significantly rescued NK cell cytotoxicity from Hp(2–20)–induced inhibition (P < 0.01–0.001, Wilcoxon’s rank sum test for pairs).
Expression of CD3ζ.
NK cells and other lymphocytes recovered from tumor-bearing animals (21) or patients with solid cancer disease, including gastric adenocarcinomas (22, 23), show a reduced expression of a critical signal-transducing molecule, CD3ζ (TcRζ). The proposed link between oxygen radical formation by monocyte/macrophages and cancer-related CD3ζ disappearance (24), along with our finding that Hp(2–20) induced oxygen radical production, encouraged us to examine the CD3ζ expression of NK cells after exposure to Hp(2–20)–activated monocytes.
A problem in the study of CD3ζ of NK cells is that the expression of characteristic surface markers on NK cells, such as CD56 or CD16, is strongly reduced by incubation with monocyte/macrophages. In contrast, T cells retain their main identification structure CD3ε after exposure to monocytes (12, 15). Therefore, an appropriate method to detect changes in CD3ζ expression is to study CD3ε– lymphocytes in NK cell–enriched lymphocyte preparations.
We found that Hp(2–20)–activated monocytes, but not monocytes treated with Hp1 (not shown), induced the disappearance of CD3ζ+ cells from viable CD3ε–, NK cell–enriched lymphocytes; more than 80% of these lymphocytes were CD56+ NK cells (Figure 5a). The inhibition was completely prevented by SOD and catalase (Figure 5b).
Figure 5.
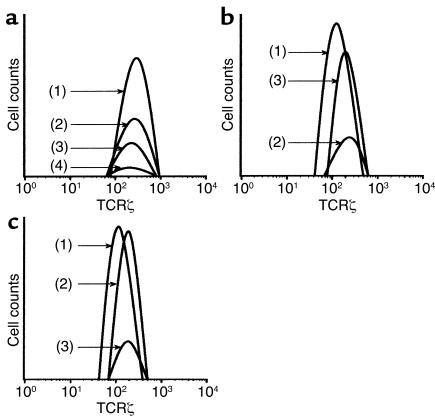
Hp(2–20) (50 μM) triggers the disappearance of the signal-transducing molecule CD3ζ from NK cells. Monocytes and/or NK cell–enriched lymphocytes were incubated as described in the legend to Figure 4. After incubation of the lymphocyte/monocyte mixture, cells in a CD3ε– lymphocyte gate (>80% of which were CD56+ NK cells) were assayed for expression of CD3ζ by use of flow cytometry. The histogram in a shows CD3ζ expression by gated CD3ε– control lymphocytes + 25% monocytes (line 1), Hp(20–20)–treated lymphocytes + 25% monocytes (line 2), control lymphocytes + 50% monocytes (line 3), and Hp(2–20)–treated lymphocytes + 50% monocytes (line 4). The histogram in b shows corresponding CD3ζ expression of control CD3ε– lymphocytes (line 1), Hp(20–20)–treated lymphocytes + 25% monocytes (line 2), and Hp(20–20)–treated lymphocytes + 25% monocytes treated with catalase and SOD (line 3). The histogram in c shows CD3ζ expression of control CD3ε– lymphocytes (line 1), Hp(20–20)–treated lymphocytes + 25% monocytes + histamine (50 μM; line 2), and Hp(20–20)–treated lymphocytes + 25% monocytes + histamine + ranitidine (50 μM; line 3). The Hp(2–20) peptide was added 20 minutes after the onset of incubation. All data are expressed as percentage of CD3ζ+ cells in a CD3ε– gate, set to comprise only viable lymphocytes. Similar results were obtained in three separate experiments.
Apoptotic cell death in NK cells and T cells.
An enhanced level of apoptosis is a common feature of lymphocytes recovered from patients with advanced gastric carcinoma (23). Morphological changes characteristic of lymphocyte apoptosis were observed after overnight incubation of lymphocytes with monocytes activated by Hp(2–20). The Hp(2–20)–induced apoptosis was observed in NK cells and in CD3ε+ T cells (Figure 6a). Apoptosis was confirmed by DNA fragmentation assay (TUNEL assay) and annexin V staining (refs. 14, 15 and not shown) and completely prevented by SOD and catalase (Figure 6b).
Figure 6.
Hp(2–20) triggers apoptosis in NK cells and T cells. Monocytes and/or NK cell–enriched lymphocytes were prepared as described in the legend to Figure 4. After incubation, cells in a lymphocyte gate were assayed for morphological features of apoptosis (reduced forward and increased right-angle scatter) by use of flow cytometry. (a) Data are the frequency of apoptotic CD56+ (NK; filled bars) or CD3ε+ (T; open bars) after the following treatments: lymphocytes incubated in culture medium (control; lane 1) lymphocytes + 25% monocytes (lane 2), lymphocytes + 25% monocytes + Hp(2–20) (50 μM; lane 3), lymphocytes + 50% monocytes (lane 4), lymphocytes + 50% monocytes + Hp(2–20) (lane 5). The inset shows apoptosis induced by Hp(2–20)–activated monocytes in all lymphocytes, and these data are the mean ± SEM of three separate experiments. The results in b show the apoptosis of lymphocytes induced by 25% monocytes (light gray bars) or 50% monocytes (dark gray bars) activated with Hp(2–20) in cell mixtures treated with SOD + catalase (cat) or histamine (his) (50 μM), alone or in the presence of the H2-receptor antagonist ranitidine (ran) (50 μM).
T cell activation.
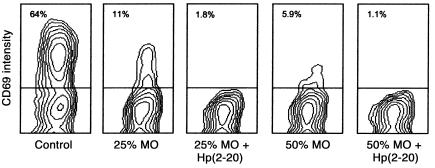
We next determined the de novo expression of the early activation antigen CD69 (Leu-23) on CD3ε+ T cells obtained from a T cell–enriched lymphocyte fraction incubated in the presence or absence of Hp(2–20)–activated monocytes. CD3ε+ T cells treated with IL-2 (100 U/ml) in the absence of monocytes acquired cell surface CD69. The IL-2–induced expression of CD69 was significantly reduced by monocytes, and the downregulation of CD69 was strongly potentiated by Hp(2–20) (Figure 7). More than 80% of NK cells acquired CD69 after treatment with IL-2 in the absence of monocytes. IL-2 weakly induced CD69 on NK cells when these cells were incubated with monocytes, and the inhibition was effectively potentiated by Hp(2–20) (not shown).
Figure 7.
Hp(2–20) triggers functional inhibition of T cells. T cell–enriched lymphocytes were treated with culture medium (control) or admixed with 25% or 50% monocytes and Hp(2–20) (50 μM), as indicated. All cells were also treated with IL-2 (100 U/ml) during overnight incubation, followed by analysis of the activation marker CD69 in gated viable (nonapoptotic) CD3ε+ lymphocytes by use of flow cytometry. Hp(2–20) did not alter the IL-2–induced acquisition of CD69 in T cells when incubated with lymphocytes without monocytes (not shown). Less than 3% of CD3ε+ T cells expressed CD69 before the addition of IL-2 or after incubation in culture medium overnight (not shown). Similar results were obtained in three separate experiments. MO, monocytes.
Histamine inhibits Hp(2–20)–induced radical production and restores lymphocyte function and viability
Effect of histamine on NADPH-oxidase activity.
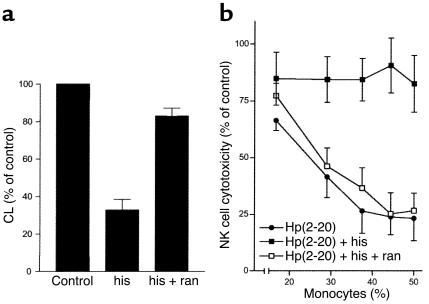
The high concentrations of histamine normally present in the gastric mucosa (approximately 10–100 μM; refs. 25, 26) led us to investigate the effects of histamine on Hp(2–20)–induced oxygen radical formation in monocytes. Histamine markedly inhibited the oxygen radical formation induced by Hp(2–20), and the specific histamine H2-receptor antagonist ranitidine reversed the inhibition (Figure 8a).
Figure 8.
Hp(2–20)–induced oxygen radical production and its inhibition by histamine. Superoxide anion production in elutriated monocytes was investigated by isoluminol-amplified CL (a). Cells were treated with histamine (50 μM) or the histamine H2-receptor antagonist ranitidine (50 μM). Data show mean values ± SEM of four separate experiments. (b) Data are NK cell cytotoxicity against K562 target cells. The cells were prepared as described in the legend to Figure 5 and treated with Hp(2–20) (50 μM; filled circles), Hp(2–20) + histamine (50 μM; filled boxes), or Hp(2–20) + histamine + ranitidine (50 μM; filled boxes). Results are expressed as percentage of control, where 100% is the cell lysis percentage of control lymphocytes without monocytes added, and are the mean ± SEM of four separate experiments.
Effect of histamine on NK cell and T cell function.
Earlier studies show that histamine maintains NK cell and T cell function in the presence of suppressive phagocytes by inhibiting oxygen radical production (14, 15). We therefore investigated whether histamine protected NK cells and T cells from monocyte-induced, Hp(2–20)–mediated suppression. Histamine prevented the following Hp(2–20)–induced events: downregulation of CD3ζ expression in NK cells/T cells (Figure 5c), triggering of NK cell and T cell apoptosis (Figure 6b), and inhibition of NK cell antitumor activity (Figure 8b). The histamine-induced protection of T cells and NK cells was antagonized by ranitidine (Figure 5c, Figure 6b, and Figure 8b).
Discussion
In this study, we show that a H. pylori–derived peptide, Hp(2–20), is a monocyte chemoattractant that also triggers NADPH-oxidase–dependent oxygen radical formation. In this regard, Hp(2–20) was as potent as previously identified H. pylori–derived proinflammatory peptides such as NAP and the peptide derived from the N-terminal portion of the bacterial urease (7–9). Monocyte activation was mediated by the FPRL1 as well as by an earlier orphan monocyte receptor, FPRL2. Several phagocyte chemoattractant receptors have been identified and characterized with respect to agonist binding, generation of intracellular signals, and cellular responses. FPRL1 (11, 27), which is the receptor shown earlier to recognize Hp(2–20) in neutrophils (11), binds to a broad spectrum of ligands that do not show any sequence homologies. FPRL1 binds LXA4 with high affinity and has been referred to as the LXA4 receptor (28). Other peptides/proteins with agonist activity at FPRL1 include a leucine zipperlike domain of the HIV-1 envelope gp41 (29), a peptide derived from the HIV-1 envelope gp120 (30), the acute-phase protein serum amyloid A (31), the mitochondrial peptide MYFINILTTL (32), LL-37, a bactericidal peptide derived from neutrophil cathelicidin (33), and the neurotoxic prion peptide fragment PrP106–126 (34). FPRL1 is thus a truly promiscuous receptor.
A third member of the FPR receptor family, FPRL2, which shows 83% amino acid sequence homology (particularly in the signaling cytoplasmic domain) with FPRL1 (35, 36), is expressed only by monocytes. No natural FPRL2 agonists have been identified. Our data demonstrate that Hp(2–20) activated monocytes also via FPRL2. It is, however, not possible to determine the relative contribution by FPRL1 and FPRL2 for the signals transduced until receptor-specific agonists/inhibitors become available. The finding that the synthetic peptide WKYMVm desensitized monocytes to a subsequent activation with Hp(2–20) strongly suggests that these agonists operate through the same receptor, but WKYMVm, in addition to being a high-affinity ligand for FPRL1, is an agonist also for FPRL2 (20). The results in this article identify Hp(2–20) as a new FPRL2 ligand, and the availability of novel agonists recognized by FPRL2 will undoubtedly be of help in screening for other agonists and determine whether FPRL2 shows the same type of promiscuity as that described for FPRL1, as well as in identifying endogenous natural ligands for this receptor.
In a second part of this study, we report that monocytes activated by Hp(2–20) induce inhibition of NK cell and T cell function. Thus, the addition of Hp(2–20) to lymphocytes and monocytes in a mixture aimed at mimicking the mononuclear cell infiltrate of H. pylori–infected gastric tissue (3, 4) triggered inhibition of NK cell–mediated antitumor cytotoxicity, inhibition of T cell inducibility by IL-2, downregulation of the CD3ζ transduction molecule expression by NK cells, and subsequent NK cell and T cell death by apoptosis. These inhibitory events were prevented by scavengers of NADPH-oxidase–derived oxygen radicals and were thus by all probability explained by the FPRL1/FPRL2–mediated oxygen radical induction by Hp(2–20).
An hypothesis emerging from these data is that the Hp(2–20) peptide may contribute to the recruitment and activation of monocyte/macrophages to the inflammatory tissue of H. pylori–infected gastric tissue. In this regard, Hp(2–20) was found to possess properties similar to those earlier described for other H. pylori–derived peptides such as a 61-kDa N-terminal portion of the bacterial urease (7) and Hp-NAP (8). Our data may also have implications for the carcinogenesis in chronically infected gastritis tissue. Two properties of Hp(2–20) may be relevant for carcinogenesis: (a) triggering of the production of monocyte-derived oxygen radicals, which are mutagenic and potentially carcinogenic (17), and (b) a secondary suppression of lymphocytes relevant to the elimination of malignant cells.
It has been suggested that monocyte/macrophages may be responsible for the dysfunction of lymphocytes, including NK cells and T cells, which is frequently observed at the site of neoplastic tumor growth (24, 37). Much attention has been focused on the defective expression of CD3ζ, a transduction molecule expressed by NK cells and T cells, in lymphocytes from cancer patients, including those with gastric adenocarcinoma (22, 23). The disappearance of CD3ζ has been attributed to oxygen radical production by monocyte/macrophages (21, 38). Our finding that Hp(2–20), by triggering oxygen radical formation in monocytes, induced the disappearance of CD3ζ from lymphocytes suggests that this peptide may contribute to lymphocyte dysfunction in gastric adenocarcinoma. It is noteworthy that changes typical for lymphocytes recovered from patients with invasive gastric carcinomas, such as CD3ζ downmodulation and apoptosis (23, 39), were induced by the Hp(2–20) peptide in vitro. However, it should be emphasized that further studies are needed to clarify the putative role of Hp(2–20) in gastric carcinogenesis.
Finally, a gastric mucosal constituent, histamine, was found to reduce or inhibit the Hp(2–20)–induced formation of oxygen radicals and thereby protect T cells and NK cells from functional inhibition and apoptotic cell death. This effect of histamine was clearly mediated by histamine H2 receptors expressed by monocytes, and concentrations of histamine similar to those detected in human gastric mucosal tissue (25, 26) were sufficient to mediate the protective effects. A tentative hypothesis emerging from these data is that the monocyte recruitment and activation induced by Hp(2–20), and presumably also by other H. pylori–derived proinflammatory peptides, may be balanced by the availability of histamine at monocyte histamine H2 receptors. Given that monocyte activation is a prominent feature of H. pylori–induced gastritis, this hypothesis is supported by the findings that ranitidine treatment, in addition to relieving acid-related symptoms, reportedly also markedly aggravates antral and corporal gastric inflammation (40). Further studies including, for example, the function and viability of gastric mucosal NK cells and T cells in H. pylori–infected subjects are required to determine the in vivo relevance of these novel mechanisms.
Acknowledgments
This study was supported by the Swedish Cancer Foundation (Cancerfonden), the Swedish Medical Research Council, and Maxim Pharmaceuticals Inc. K. Hellstrand is a fellow of the Swedish Cancer Society.
References
- 1.Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35:90–97. [PubMed] [Google Scholar]
- 2.Ebert MP, Yu J, Sung JJ, Malfertheiner P. Molecular alterations in gastric cancer: the role of Helicobacter pylori. Eur J Gastroenterol Hepatol. 2000;12:795–798. doi: 10.1097/00042737-200012070-00013. [DOI] [PubMed] [Google Scholar]
- 3.Agnihotri N, et al. Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand J Gastroenterol. 1998;33:704–709. doi: 10.1080/00365529850171639. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Andersson EM, Helander HF. Reactions from rat gastric mucosa during one year of Helicobacter pylori infection. Dig Dis Sci. 1999;44:116–124. doi: 10.1023/a:1026662402734. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi H, et al. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol. 2001;96:574–578. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishigami S, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 7.Mai UE, et al. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satin B, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen LA. Modulating phagocyte activation: the pros and cons of Helicobacter pylori virulence factors. J Exp Med. 2000;191:1451–1454. doi: 10.1084/jem.191.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putsep K, Branden CI, Boman HG, Normark S. Antibacterial peptide from H. pylori. Nature. 1999;398:671–672. doi: 10.1038/19439. [DOI] [PubMed] [Google Scholar]
- 11.Bylund J, et al. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob Agents Chemother. 2001;45:1700–1704. doi: 10.1128/AAC.45.6.1700-1704.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- 13.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 14.Mellqvist UH, et al. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96:1961–1968. [PubMed] [Google Scholar]
- 15.Hansson M, et al. Histamine protects T cells and natural killer cells against oxidative stress. J Interferon Cytokine Res. 1999;19:1135–1144. doi: 10.1089/107999099313073. [DOI] [PubMed] [Google Scholar]
- 16.Boman HG. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff, S.J. 1999. Oxygen metabolites from phagocytes. In Inflammation: basic principles and clinical correlates. Volume 1. 3rd edition. J.I. Gallin and R. Snyderman, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 721–768.
- 18.O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putsep K, Normark S, Boman HG. The origin of cecropins; implications from synthetic peptides derived from ribosomal protein L1. FEBS Lett. 1999;451:249–252. doi: 10.1016/s0014-5793(99)00582-7. [DOI] [PubMed] [Google Scholar]
- 20.Christophe T, et al. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/LXA4R and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 21.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881–1886. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kono K, Ichihara F, Iizuka H, Sekikawa T, Matsumoto Y. Expression of signal transducing T-cell receptor zeta molecules after adoptive immunotherapy in patients with gastric and colon cancer. Int J Cancer. 1998;78:301–305. doi: 10.1002/(SICI)1097-0215(19981029)78:3<301::AID-IJC7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi A, et al. Elevated caspase-3 activity in peripheral blood T cells coexists with increased degree of T-cell apoptosis and down-regulation of TCR zeta molecules in patients with gastric cancer. Clin Cancer Res. 2001;7:74–80. [PubMed] [Google Scholar]
- 24.Kiessling R, et al. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bechi P, et al. Reflux-related gastric mucosal injury is associated with increased mucosal histamine content in humans. Gastroenterology. 1993;104:1057–1063. doi: 10.1016/0016-5085(93)90274-g. [DOI] [PubMed] [Google Scholar]
- 26.Lonroth H, Rosengren E, Olbe L, Lundell L. Histamine metabolism in human gastric mucosa. Effect of pentagastrin stimulation. Gastroenterology. 1990;98:921–928. doi: 10.1016/0016-5085(90)90016-t. [DOI] [PubMed] [Google Scholar]
- 27.Bao L, Gerard NP, Eddy RL, Jr, Shows TB, Gerard C. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 28.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su SB, et al. T21/DP107, A synthetic leucine zipper-like domain of the HIV-1 envelope gp41, attracts and activates human phagocytes by using G-protein- coupled formyl peptide receptors. J Immunol. 1999;162:5924–5930. [PubMed] [Google Scholar]
- 30.Deng X, et al. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein- coupled receptor FPRL1/LXA4R. Blood. 1999;94:1165–1173. [PubMed] [Google Scholar]
- 31.Su SB, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Y, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Y, et al. The neurotoxic prion peptide fragment PrP(106–126) is a chemotactic agonist for the G protein-coupled receptor formyl peptide receptor-like 1. J Immunol. 2001;166:1448–1451. doi: 10.4049/jimmunol.166.3.1448. [DOI] [PubMed] [Google Scholar]
- 35.Durstin M, Gao JL, Tiffany HL, McDermott D, Murphy PM. Differential expression of members of the N-formylpeptide receptor gene cluster in human phagocytes. Biochem Biophys Res Commun. 1994;201:174–179. doi: 10.1006/bbrc.1994.1685. [DOI] [PubMed] [Google Scholar]
- 36.Ye RD, Boulay F. Structure and function of leukocyte chemoattractant receptors. Adv Pharmacol. 1997;39:221–289. doi: 10.1016/s1054-3589(08)60073-3. [DOI] [PubMed] [Google Scholar]
- 37.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 38.Nakagomi H, et al. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 39.Kim CW, et al. Alteration of signal-transducing molecules and phenotypical characteristics in peripheral blood lymphocytes from gastric carcinoma patients. Pathobiology. 1999;67:123–128. doi: 10.1159/000028061. [DOI] [PubMed] [Google Scholar]
- 40.Meining A, Bosseckert H, Caspary WF, Nauert C, Stolte M. H2-receptor antagonists and antacids have an aggravating effect on Helicobacter pylori gastritis in duodenal ulcer patients. Aliment Pharmacol Ther. 1997;11:729–734. doi: 10.1046/j.1365-2036.1997.00196.x. [DOI] [PubMed] [Google Scholar]