Abstract
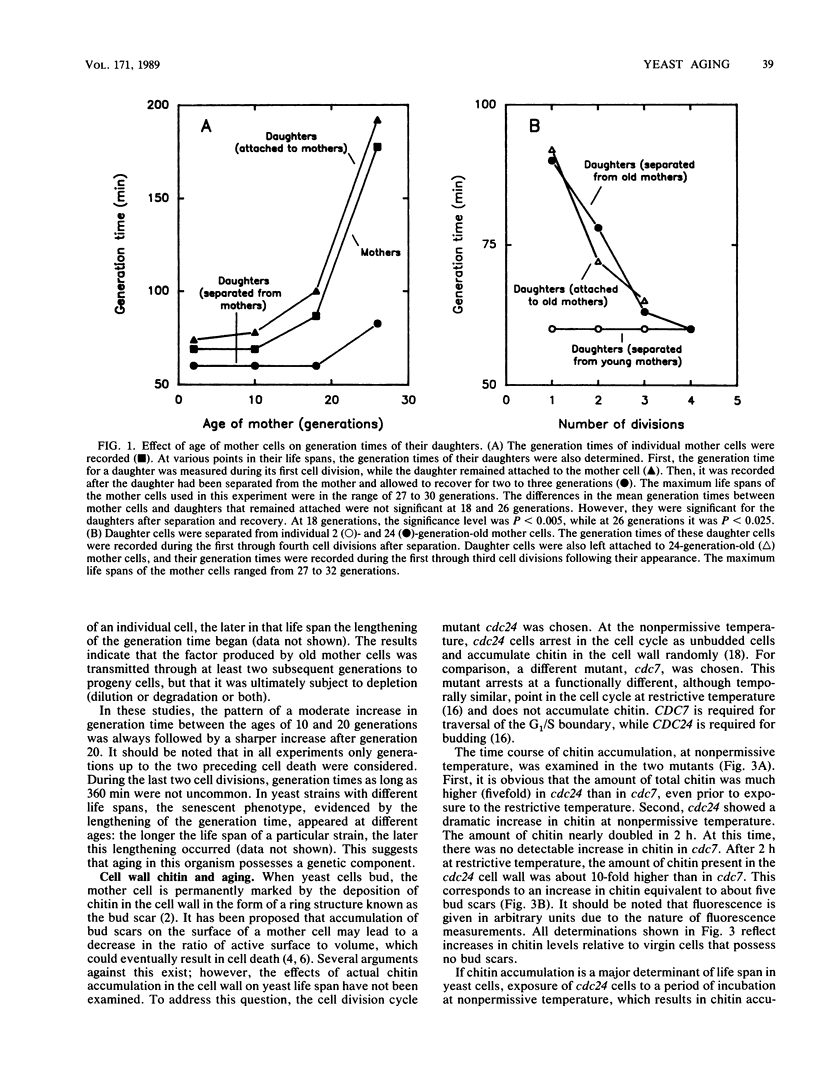
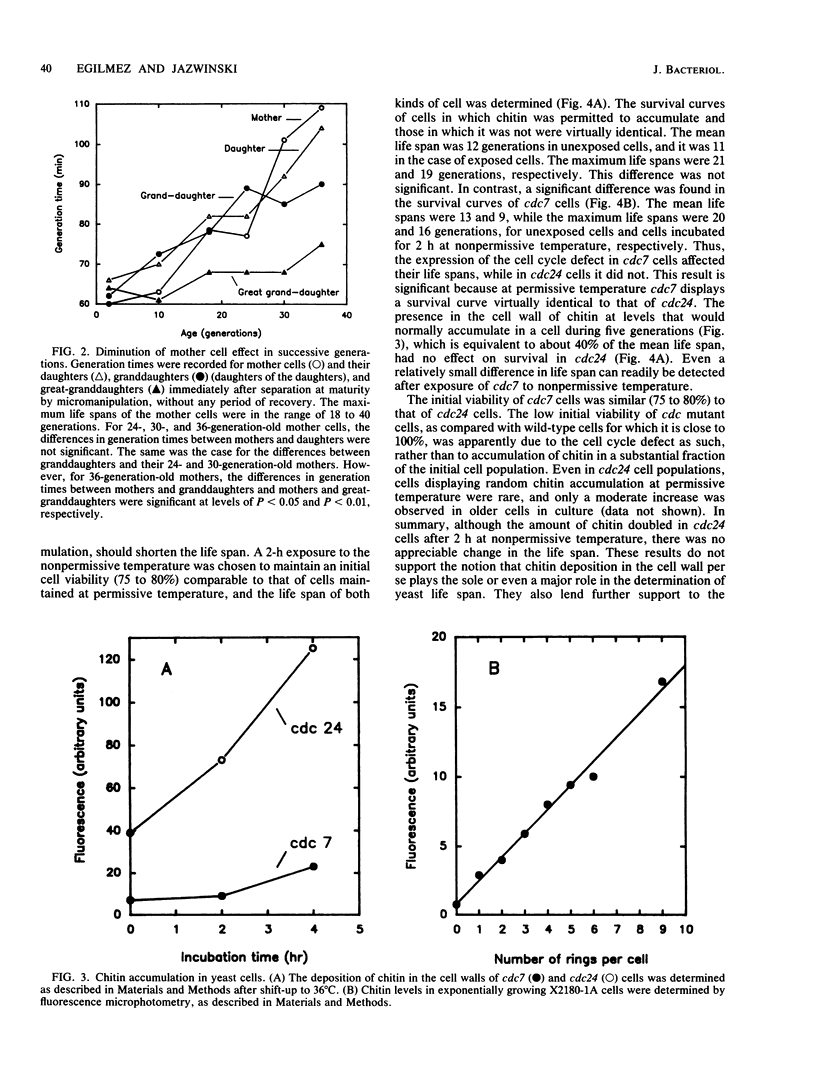
The life spans of individual Saccharomyces cerevisiae cells were determined microscopically by counting the number of buds produced by each cell to provide a measure of the number of cell generations (age) before death. As the cells aged, their generation times increased five- to sixfold. The generation times of daughter cells were virtually identical to those of their mothers throughout the life spans of the mothers. However, within two to three cell divisions after the daughters were detached from their mothers by micromanipulation, their generation times reverted to that characteristic of their own age. Recovery from the mother cell effect was also observed when the daughters were left attached to their mothers. The results suggest that senescence, as manifested by the increase in generation time, is a phenotypically dominant feature in yeast cells and that it is determined by a diffusible cytoplasmic factor(s) that undergoes turnover. This factor(s) appeared to be transmitted by a cell not only to its daughter, but also indirectly to its granddaughter. In separate studies, it was determined that the induced deposition of chitin, the major component of the bud scar, in the yeast cell wall had no appreciable effect on life span. We raise the possibility that the cytoplasmic factor(s) that appears to mediate the "senescent phenotype" is a major determinant of yeast life span. This factor(s) may be the product of age-specific gene expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHOLOMEW J. W., MITTWER T. Demonstration of yeast bud scars with the electron microscope. J Bacteriol. 1953 Mar;65(3):272–275. doi: 10.1128/jb.65.3.272-275.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. A molecular model for morphogenesis: the primary septum of yeast. Curr Top Cell Regul. 1974;8(0):1–32. doi: 10.1016/b978-0-12-152808-9.50008-0. [DOI] [PubMed] [Google Scholar]
- Jamet-Vierny C., Begel O., Belcour L. Senescence in Podospora anserina: amplification of a mitochondrial DNA sequence. Cell. 1980 Aug;21(1):189–194. doi: 10.1016/0092-8674(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Johnston J. R. Reproductive capacity and mode of death of yeast cells. Antonie Van Leeuwenhoek. 1966;32(1):94–98. doi: 10.1007/BF02097448. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Pereira-Smith O. M., Smith J. R. Existence of high abundance antiproliferative mRNA's in senescent human diploid fibroblasts. Science. 1986 Apr 18;232(4748):393–395. doi: 10.1126/science.2421407. [DOI] [PubMed] [Google Scholar]
- MORTIMER R. K., JOHNSTON J. R. Life span of individual yeast cells. Nature. 1959 Jun 20;183(4677):1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- Müller I. Experiments on ageing in single cells of Saccharomyces cerevisiae. Arch Mikrobiol. 1971;77(1):20–25. doi: 10.1007/BF00407985. [DOI] [PubMed] [Google Scholar]
- Müller I. Parental age and the life-span of zygotes of Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 1985;51(1):1–10. doi: 10.1007/BF00444223. [DOI] [PubMed] [Google Scholar]
- Müller I., Zimmermann M., Becker D., Flömer M. Calendar life span versus budding life span of Saccharomyces cerevisiae. Mech Ageing Dev. 1980 Jan;12(1):47–52. doi: 10.1016/0047-6374(80)90028-7. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Shore D. Transcriptional regulation in the yeast life cycle. Science. 1987 Sep 4;237(4819):1162–1170. doi: 10.1126/science.3306917. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olashaw N. E., Kress E. D., Cristofalo V. J. Thymidine triphosphate synthesis in senescent WI38 cells. Relationship to loss of replicative capacity. Exp Cell Res. 1983 Dec;149(2):547–554. doi: 10.1016/0014-4827(83)90365-8. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Fisher S. F., Smith J. R. Senescent and quiescent cell inhibitors of DNA synthesis. Membrane-associated proteins. Exp Cell Res. 1985 Oct;160(2):297–306. doi: 10.1016/0014-4827(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Pohley H. J. A formal mortality analysis for populations of unicellular organisms (Saccharomyces cerevisiae). Mech Ageing Dev. 1987 May;38(3):231–243. doi: 10.1016/0047-6374(87)90092-3. [DOI] [PubMed] [Google Scholar]
- Sloat B. F., Pringle J. R. A mutant of yeast defective in cellular morphogenesis. Science. 1978 Jun 9;200(4346):1171–1173. doi: 10.1126/science.349694. [DOI] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]


