Abstract
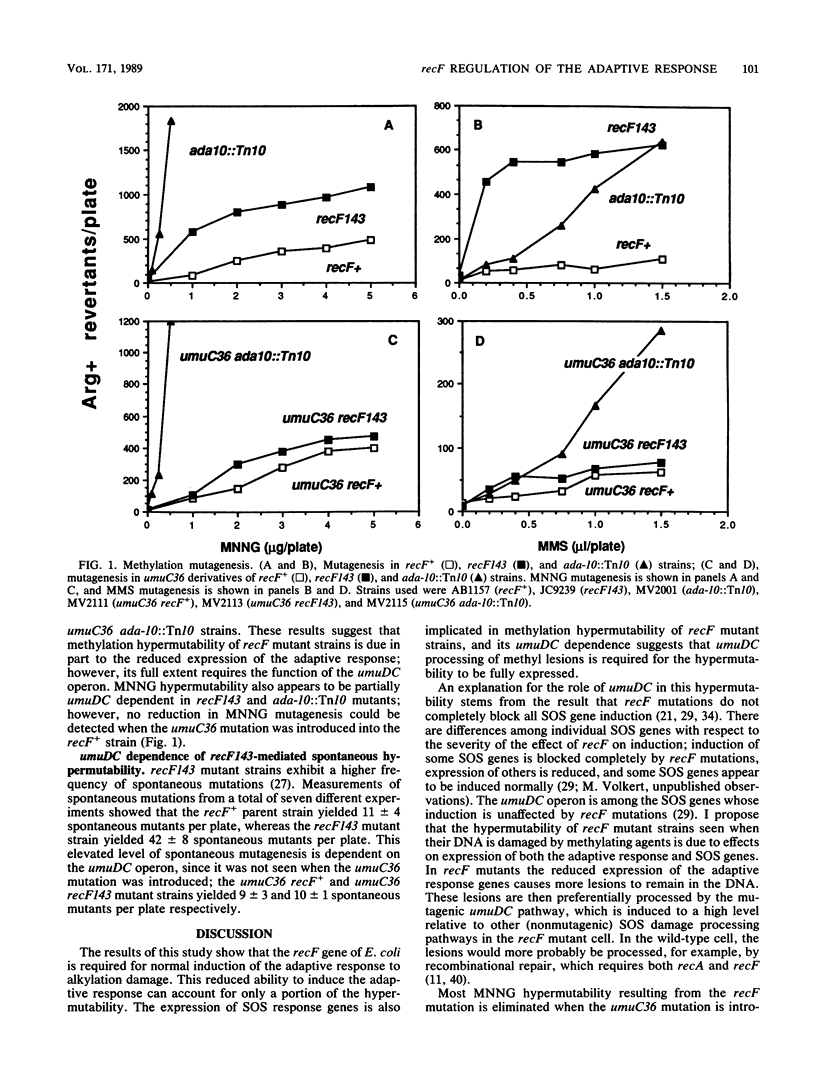
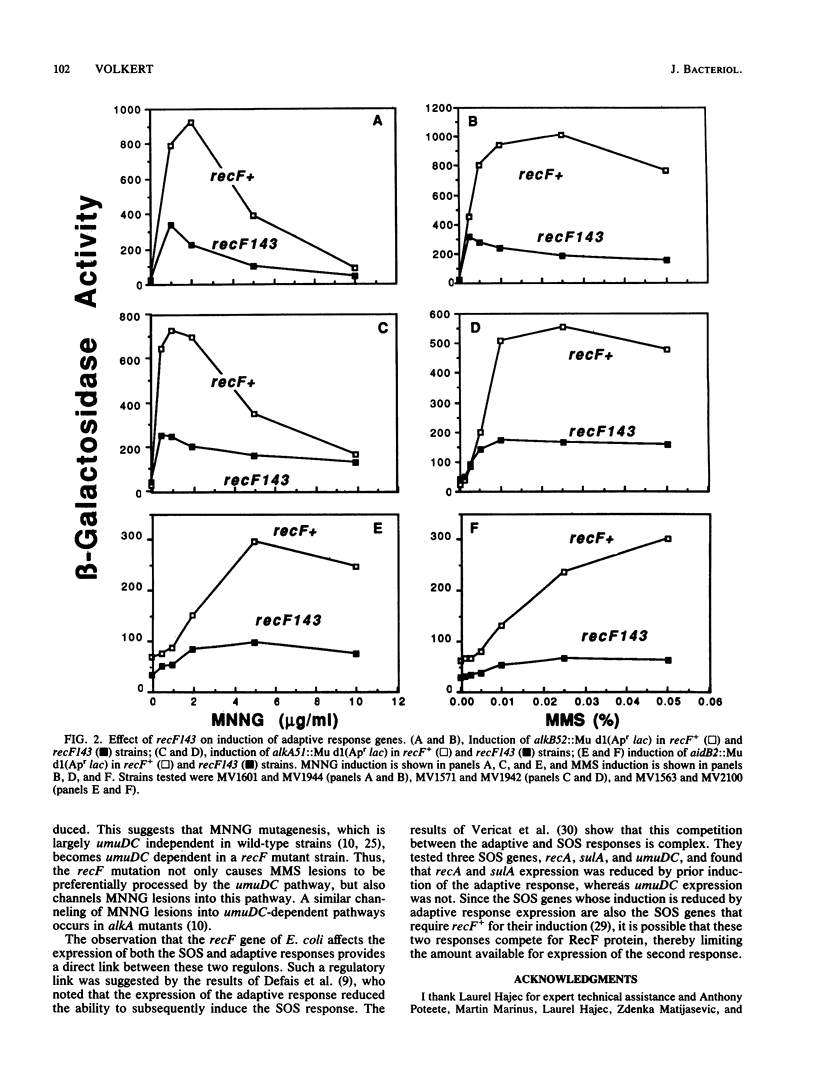
Escherichia coli recF mutants are hypermutable when treated with methyl methanesulfonate (G. C. Walker, Mol. Gen. Genet. 152:93-103, 1977). In this study, methylation hypermutability of recF mutant strains was examined, and it was found that recF+ is required for normal induction of the adaptive response to alkylation damage. Although this regulatory effect of recF mutations results in reduced levels of enzymes that specifically repair methyl lesions in DNA, it only partially explains the hypermutability. Further examination showed that methylation hypermutability of recF mutant strains required a functional umuDC operon, a component of the SOS response. These results lead to the hypothesis that methylation hypermutability results from the effects of recF mutations on the induction of both the SOS response and the adaptive response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armengod M. E., Blanco M. Influence of the recF143 mutation of Escherichia coli K12 on prophage lambda induction. Mutat Res. 1978 Oct;52(1):37–47. doi: 10.1016/0027-5107(78)90093-3. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Sandler S. J., Armengod M. E., Ream L. W., Clark A. J. Molecular analysis of the recF gene of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4622–4626. doi: 10.1073/pnas.81.15.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla Z., O'Brien P., Clark A. J. Genetic analysis of UV mutagenesis of the Escherichia coli glyU gene. Mol Gen Genet. 1987 Apr;207(1):1–8. doi: 10.1007/BF00331483. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Volkert M. R., Margossian L. J. A role for recF in repair of UV damage to DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):887–892. doi: 10.1101/sqb.1979.043.01.096. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Volkert M. R., Margossian L. J., Nagaishi H. Effects of a recA operator mutation on mutant phenotypes conferred by lexA and recF mutations. Mutat Res. 1982 Nov;106(1):11–26. doi: 10.1016/0027-5107(82)90187-7. [DOI] [PubMed] [Google Scholar]
- Cohen A., Laban A. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol Gen Genet. 1983;189(3):471–474. doi: 10.1007/BF00325911. [DOI] [PubMed] [Google Scholar]
- Defais M., Jeggo P., Samson L., Schendel P. F. Effect of the adaptive response on the induction of the SOS pathway in E. coli K-12. Mol Gen Genet. 1980;177(4):653–659. doi: 10.1007/BF00272676. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E. Induction of transversion mutations in Escherichia coli by N-methyl-N'-nitro-N-nitrosoguanidine is SOS dependent. J Bacteriol. 1985 Jul;163(1):213–220. doi: 10.1128/jb.163.1.213-220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C. The effect of lexA and recF mutations on post-replication repair and DNA synthesis in Escherichia coli K-12. Mol Gen Genet. 1975 Dec 1;141(3):189–205. doi: 10.1007/BF00341799. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982 Sep 25;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Jeggo P. Isolation and characterization of Escherichia coli K-12 mutants unable to induce the adaptive response to simple alkylating agents. J Bacteriol. 1979 Sep;139(3):783–791. doi: 10.1128/jb.139.3.783-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Rothman R. H., Clark A. J. Analysis of the role of recombination and repair in mutagenesis of Escherichia coli by UV irradiation. Genetics. 1977 Sep;87(1):1–18. doi: 10.1093/genetics/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D. Some chemical aspects of dose-response relationships in alkylation mutagenesis. Mutat Res. 1974 Jun;23(3):283–295. doi: 10.1016/0027-5107(74)90102-x. [DOI] [PubMed] [Google Scholar]
- Lemotte P. K., Walker G. C. Induction and autoregulation of ada, a positively acting element regulating the response of Escherichia coli K-12 to methylating agents. J Bacteriol. 1985 Mar;161(3):888–895. doi: 10.1128/jb.161.3.888-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- McPartland A., Green L., Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980 Jul;20(3):731–737. doi: 10.1016/0092-8674(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Sekiguchi M. Regulatory mechanisms for induction of synthesis of repair enzymes in response to alkylating agents: ada protein acts as a transcriptional regulator. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6297–6301. doi: 10.1073/pnas.83.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. The dependence of postreplication repair on uvrB in a recF mutant of Escherichia coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):279–286. doi: 10.1007/BF00272806. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Margossian L. J., Clark A. J. W-reactivation of phage lambda in recF, recL, uvrA, and uvrB mutants of E. coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):279–287. doi: 10.1007/BF00382274. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Defais M. The role of umuC gene product in mutagenesis by simple alkylating agents. Mol Gen Genet. 1980;177(4):661–665. doi: 10.1007/BF00272677. [DOI] [PubMed] [Google Scholar]
- Singer B. Mutagenesis from a chemical perspective: nucleic acid reactions, repair, translation, and transcription. Basic Life Sci. 1982;20:1–42. doi: 10.1007/978-1-4613-3476-7_1. [DOI] [PubMed] [Google Scholar]
- Southworth M. W., Bridges B. A. Influence of recF on spontaneous mutation in Escherichia coli. Mutat Res. 1984 Jun-Jul;140(2-3):67–69. doi: 10.1016/0165-7992(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Kilpatrick M. W., McCarthy T. V., Lindahl T. The intracellular signal for induction of resistance to alkylating agents in E. coli. Cell. 1986 Apr 25;45(2):315–324. doi: 10.1016/0092-8674(86)90396-x. [DOI] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J Bacteriol. 1987 Apr;169(4):1731–1736. doi: 10.1128/jb.169.4.1731-1736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vericat J. A., Guerrero R., Barbé J. Inhibition of the SOS response of Escherichia coli by the Ada protein. J Bacteriol. 1988 Mar;170(3):1354–1359. doi: 10.1128/jb.170.3.1354-1359.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R. Adaptive response of Escherichia coli to alkylation damage. Environ Mol Mutagen. 1988;11(2):241–255. doi: 10.1002/em.2850110210. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Hartke M. A. Effects of the Escherichia coli recF suppressor mutation, recA801, on recF-dependent DNA-repair associated phenomena. Mutat Res. 1987 Nov;184(3):181–186. doi: 10.1016/0167-8817(87)90015-0. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Hartke M. A. Suppression of Escherichia coli recF mutations by recA-linked srfA mutations. J Bacteriol. 1984 Feb;157(2):498–506. doi: 10.1128/jb.157.2.498-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Margossian L. J., Clark A. J. Two-component suppression of recF143 by recA441 in Escherichia coli K-12. J Bacteriol. 1984 Nov;160(2):702–705. doi: 10.1128/jb.160.2.702-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Nguyen D. C., Beard K. C. Escherichia coli gene induction by alkylation treatment. Genetics. 1986 Jan;112(1):11–26. doi: 10.1093/genetics/112.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Nguyen D. C. Induction of specific Escherichia coli genes by sublethal treatments with alkylating agents. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4110–4114. doi: 10.1073/pnas.81.13.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Plasmid (pKM101)-mediated enhancement of repair and mutagenesis: dependence on chromosomal genes in Escherichia coli K-12. Mol Gen Genet. 1977 Mar 28;152(1):93–103. doi: 10.1007/BF00264945. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Smith K. C. Mechanisms for recF-dependent and recB-dependent pathways of postreplication repair in UV-irradiated Escherichia coli uvrB. J Bacteriol. 1983 Dec;156(3):1093–1098. doi: 10.1128/jb.156.3.1093-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


