Abstract
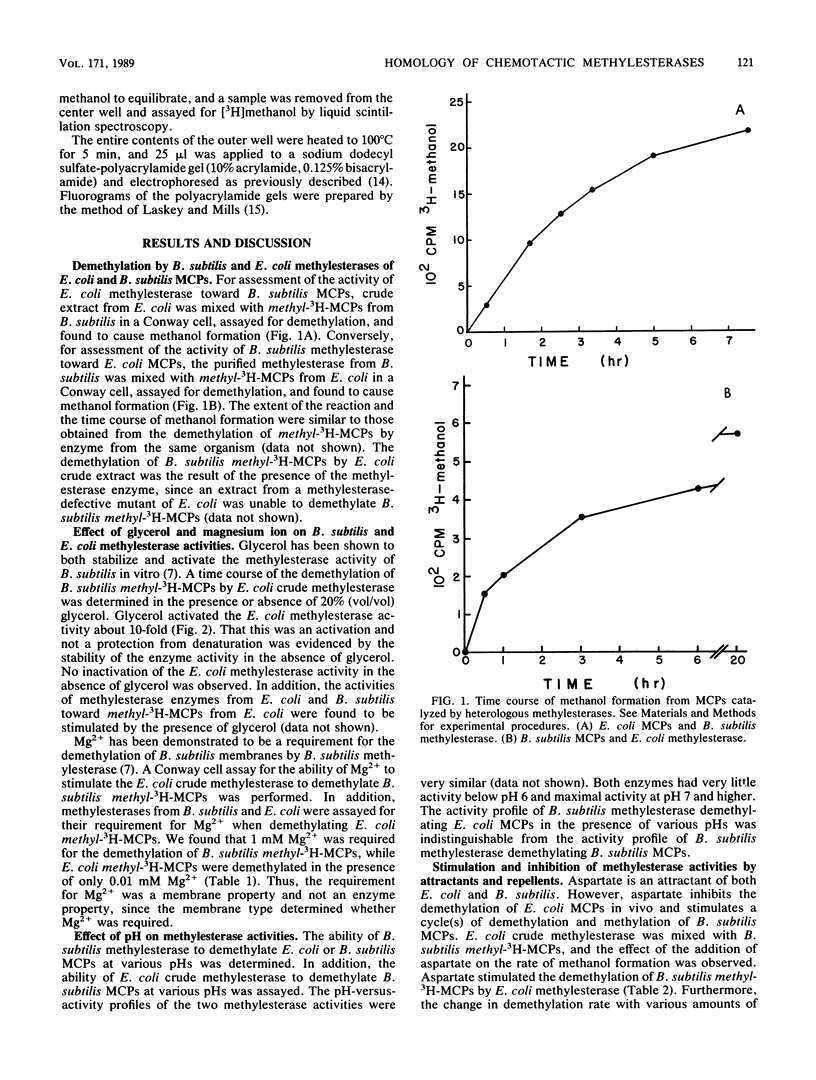
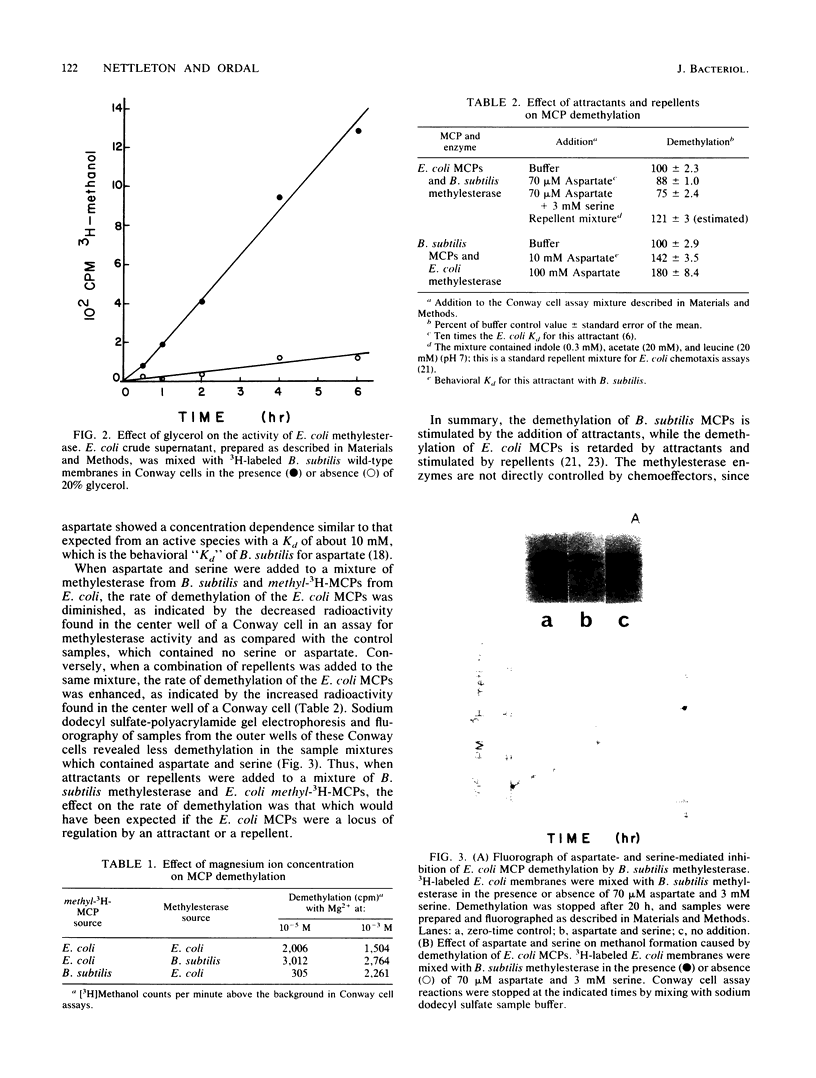
The methylesterase enzyme from Bacillus subtilis was compared with that from Escherichia coli. Both enzymes were able to demethylate methyl-accepting chemotaxis proteins (MCPs) from the other organism and were similarly affected by variations in glycerol, magnesium ion, or pH. When attractants were added to a mixture of B. subtilis MCPs and E. coli methylesterase, the rate of demethylation was enhanced. Conversely, when attractants were added to a mixture of E. coli MCPs and B. subtilis methylesterase, the rate of demethylation was diminished. These effects are what would be expected if, in these in vitro systems, the MCPs determined the rate of demethylation. These data suggest that, although the enzymes are from evolutionarily divergent organisms and are different in size, they have considerable functional homology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlgren J. A., Ordal G. W. Methyl esterification of glutamic acid residues of methyl-accepting chemotaxis proteins in Bacillus subtilis. Biochem J. 1983 Sep 1;213(3):759–763. doi: 10.1042/bj2130759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedale W. A., Nettleton D. O., Sopata C. S., Thoelke M. S., Ordal G. W. Evidence for methyl group transfer between the methyl-accepting chemotaxis proteins in Bacillus subtilis. J Bacteriol. 1988 Jan;170(1):223–227. doi: 10.1128/jb.170.1.223-227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Cassler A., Ordal G. W. Functional homology of Bacillus subtilis methyltransferase II and Escherichia coli cheR protein. J Biol Chem. 1982 Nov 10;257(21):12835–12838. [PubMed] [Google Scholar]
- Burgess-Cassler A., Ullah A. H., Ordal G. W. Purification and characterization of Bacillus subtilis methyl-accepting chemotaxis protein methyltransferase II. J Biol Chem. 1982 Jul 25;257(14):8412–8417. [PubMed] [Google Scholar]
- Chet I., Mitchell R. Ecological aspects of microbial chemotactic behavior. Annu Rev Microbiol. 1976;30:221–239. doi: 10.1146/annurev.mi.30.100176.001253. [DOI] [PubMed] [Google Scholar]
- Clarke S., Koshland D. E., Jr Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem. 1979 Oct 10;254(19):9695–9702. [PubMed] [Google Scholar]
- Goldman D. J., Nettleton D. O., Ordal G. W. Purification and characterization of chemotactic methylesterase from Bacillus subtilis. Biochemistry. 1984 Feb 14;23(4):675–680. doi: 10.1021/bi00299a014. [DOI] [PubMed] [Google Scholar]
- Goldman D. J., Ordal G. W. Sensory adaptation and deadaptation by Bacillus subtilis. J Bacteriol. 1981 Jul;147(1):267–270. doi: 10.1128/jb.147.1.267-270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. J., Worobec S. W., Siegel R. B., Hecker R. V., Ordal G. W. Chemotaxis in Bacillus subtilis: effects of attractants on the level of methylation of methyl-accepting chemotaxis proteins and the role of demethylation in the adaptation process. Biochemistry. 1982 Mar 2;21(5):915–920. doi: 10.1021/bi00534a016. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S. J., Toews M. L., Adler J. Isolation of glutamic acid methyl ester from an Escherichia coli membrane protein involved in chemotaxis. J Biol Chem. 1977 May 25;252(10):3214–3218. [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nowlin D. M., Nettleton D. O., Ordal G. W., Hazelbauer G. L. Chemotactic transducer proteins of Escherichia coli exhibit homology with methyl-accepting proteins from distantly related bacteria. J Bacteriol. 1985 Jul;163(1):262–266. doi: 10.1128/jb.163.1.262-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Gibson K. J. Chemotaxis toward amino acids by Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):151–155. doi: 10.1128/jb.129.1.151-155.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Gibson K. J. Amino acid chemoreceptors of Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):156–165. doi: 10.1128/jb.129.1.156-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Gallin J. I. Biochemistry of phagocyte chemotaxis. Curr Top Cell Regul. 1979;15:203–261. doi: 10.1016/b978-0-12-152815-7.50010-7. [DOI] [PubMed] [Google Scholar]
- Sherris D., Parkinson J. S. Posttranslational processing of methyl-accepting chemotaxis proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6051–6055. doi: 10.1073/pnas.78.10.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoelke M. S., Bedale W. A., Nettleton D. O., Ordal G. W. Evidence for an intermediate methyl-acceptor for chemotaxis in Bacillus subtilis. J Biol Chem. 1987 Feb 25;262(6):2811–2816. [PubMed] [Google Scholar]
- Toews M. L., Adler J. Methanol formation in vivo from methylated chemotaxis proteins in Escherichia coli. J Biol Chem. 1979 Mar 25;254(6):1761–1764. [PubMed] [Google Scholar]
- Toews M. L., Goy M. F., Springer M. S., Adler J. Attractants and repellents control demethylation of methylated chemotaxis proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5544–5548. doi: 10.1073/pnas.76.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf P., Koshland D. E., Jr Identification of a gamma-glutamyl methyl ester in bacterial membrane protein involved in chemotaxis. J Biol Chem. 1977 Apr 25;252(8):2793–2795. [PubMed] [Google Scholar]



