Abstract
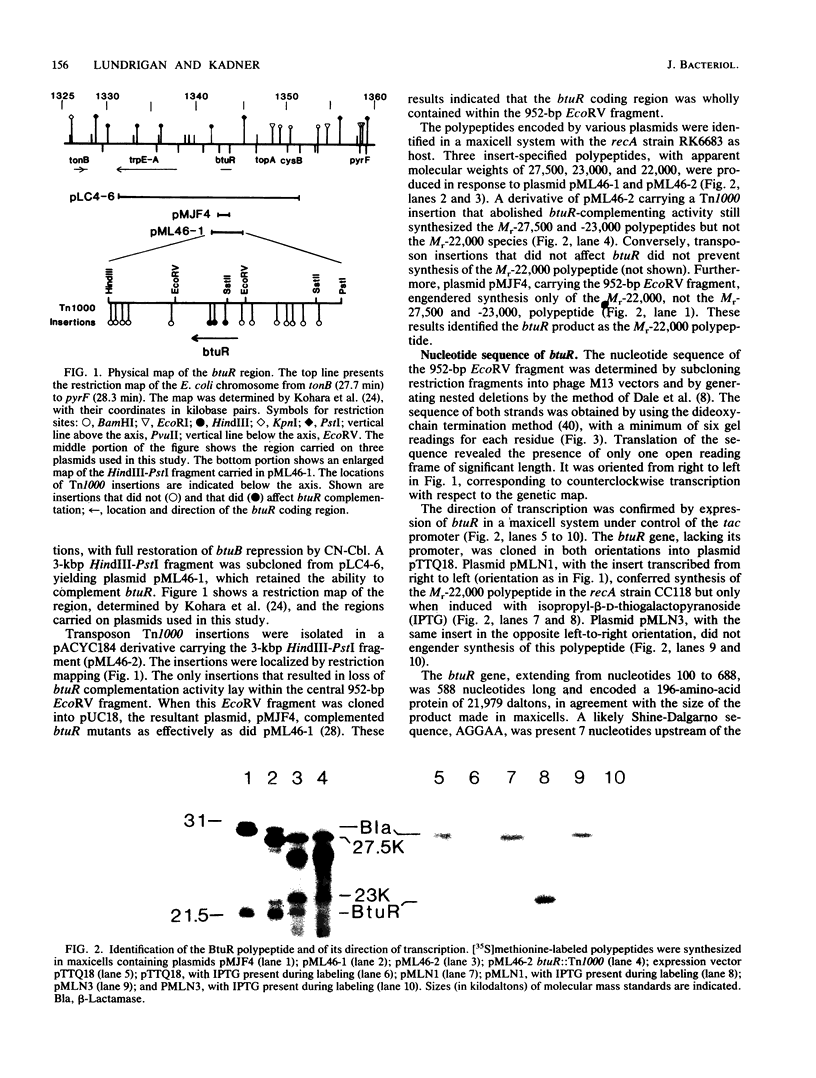
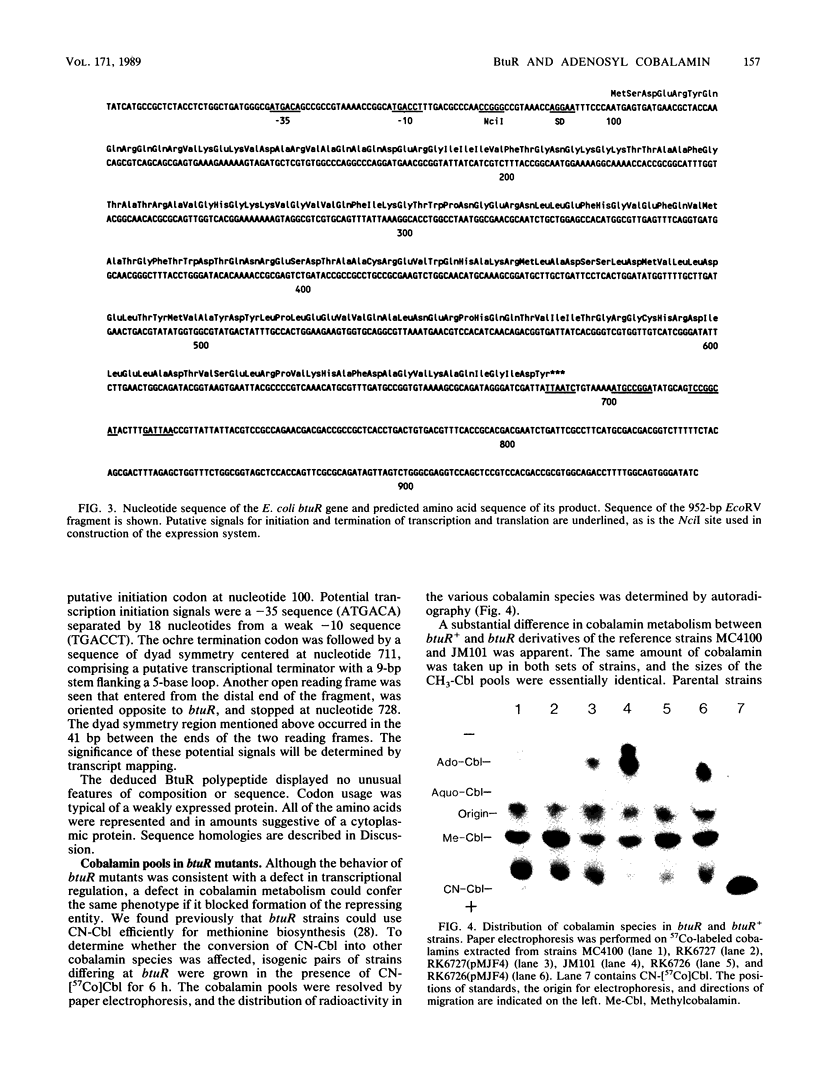
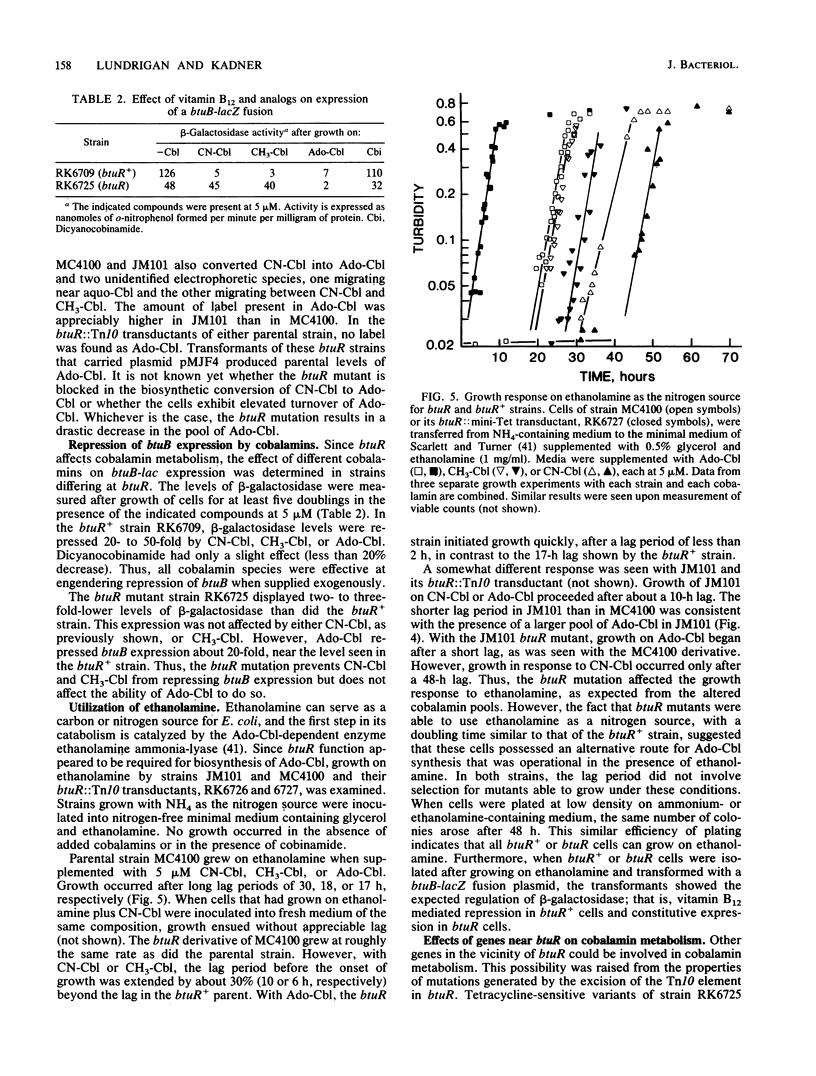
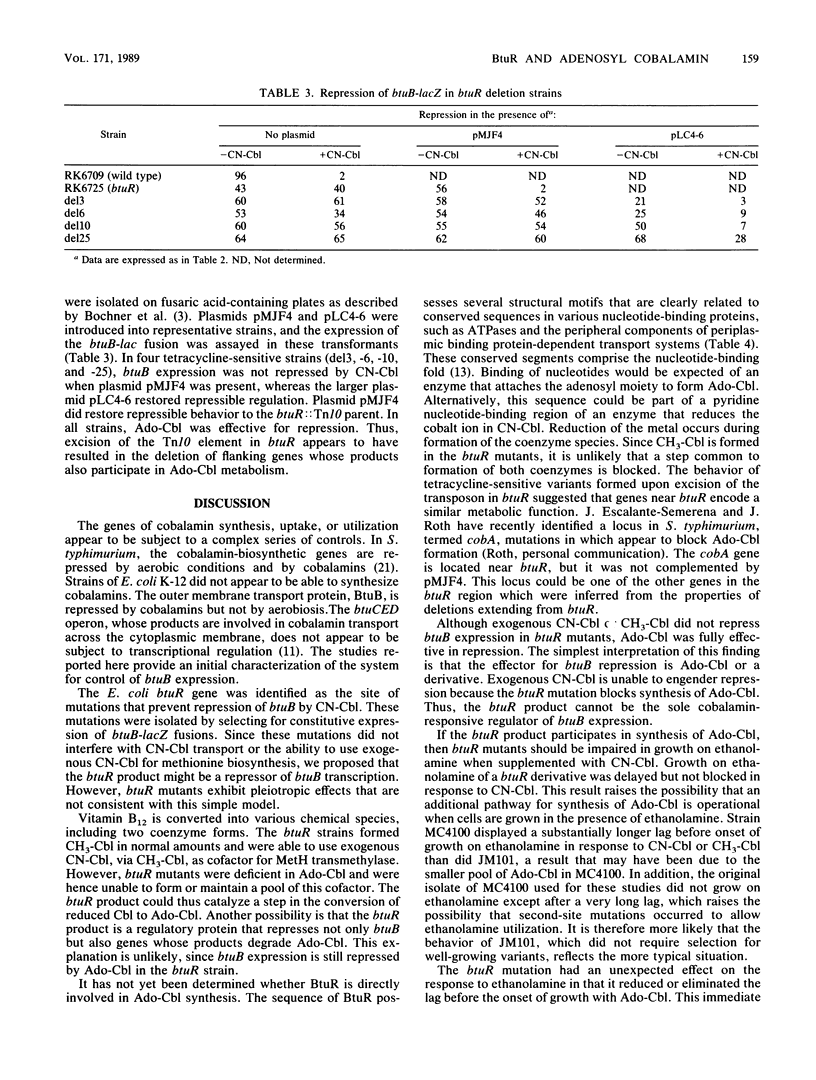
Synthesis of the Escherichia coli outer membrane protein BtuB, which mediates the binding and transport of vitamin B12, is repressed when cells are grown in the presence of vitamin B12. Expression of btuB-lacZ fusions was also found to be repressed, and selection for constitutive production of beta-galactosidase in the presence of vitamin B12 yielded mutations at btuR. The btuR locus, at 27.9 min on the chromosome map, was isolated on a 952-base-pair EcoRV fragment, and its nucleotide sequence was determined. The BtuR protein was identified in maxicells as a 22,000-dalton polypeptide, as predicted from the nucleotide sequence. Strains mutant at btuR had negligible pools of adenosylcobalamin but did convert vitamin B12 into other derivatives. Although btuB expression in a btuR strain could not be repressed by cyano- or methylcobalamin, it was repressed by adenosylcobalamin. Growth on ethanolamine as the sole nitrogen source requires adenosylcobalamin. btuR mutants grew on ethanolamine but were affected in the length of the lag period before initiation of growth, which suggested that an alternative route for adenosylcobalamin synthesis might exist. No mutations were found that conferred constitutive btuB expression in the presence of adenosylcobalamin. Other genes near btuR may also be involved in cobalamin metabolism, as suggested from the complementation behavior of strains generated by excision of the Tn10 element in btuR. These results indicated that the btuR product is involved in the metabolism of adenosylcobalamin and that this cofactor, or some derivative, controls btuB expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufrère R., Tempête M., Bohin J. P. Regulation of expression of the gene for vitamin B12 receptor cloned on a multicopy plasmid in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):358–365. doi: 10.1007/BF00430451. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Gerlach P., Middendorf A. Double negative and positive control of tsx expression in Escherichia coli. J Bacteriol. 1988 Jan;170(1):108–116. doi: 10.1128/jb.170.1.108-116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauthen S. E., Foster M. A., Woods D. D. Methionine synthesis by extracts of Salmonella typhimurium. Biochem J. 1966 Feb;98(2):630–635. doi: 10.1042/bj0980630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- DeVeaux L. C., Kadner R. J. Transport of vitamin B12 in Escherichia coli: cloning of the btuCD region. J Bacteriol. 1985 Jun;162(3):888–896. doi: 10.1128/jb.162.3.888-896.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., McCloskey J., Kersten W., Kersten H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
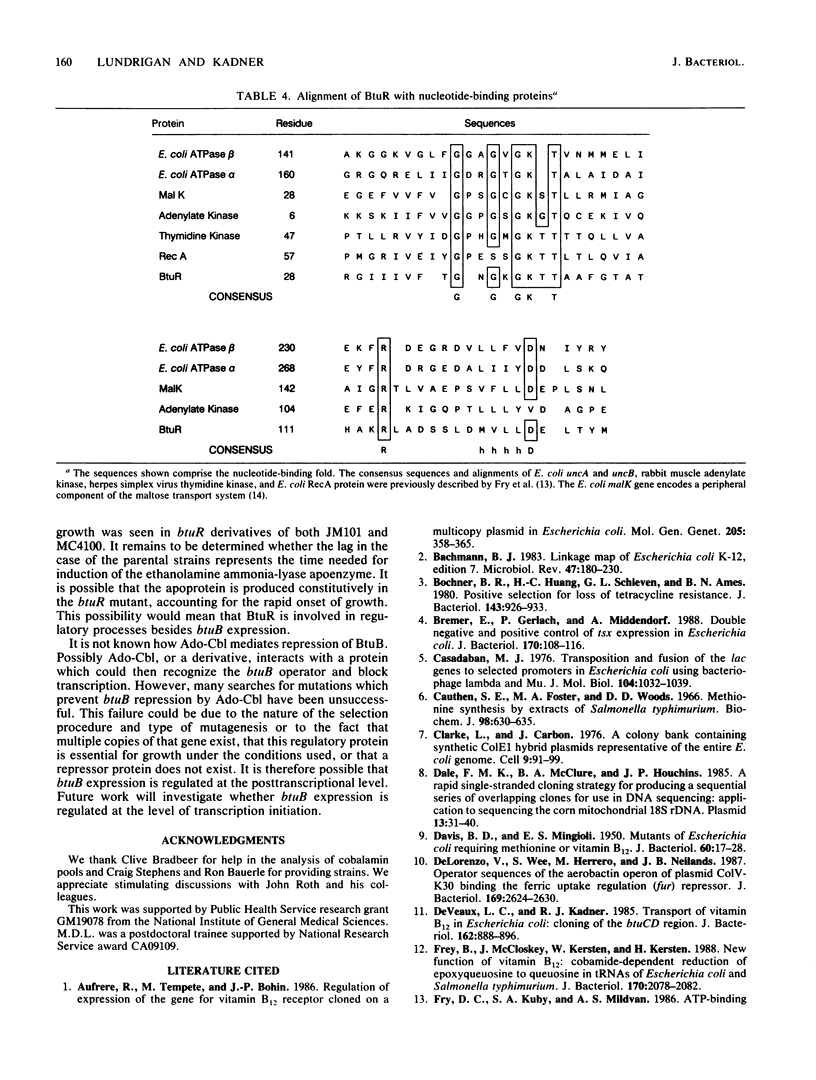
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Williams R. D., Kung H. F., Spears C., Weissbach H. Effect of methionine and vitamin B-12 on the activities of methionine biosynthetic enzymes in metJ mutants of Escherichia coli K12. Arch Biochem Biophys. 1973 Sep;158(1):249–256. doi: 10.1016/0003-9861(73)90619-x. [DOI] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Mann B. J., Kadner R. J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Roth J. R. Cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J Bacteriol. 1987 Jul;169(7):3189–3198. doi: 10.1128/jb.169.7.3189-3198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Liggins G. L. Transport of vitamin B12 in Escherichia coli: genetic studies. J Bacteriol. 1973 Aug;115(2):514–521. doi: 10.1128/jb.115.2.514-521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1050–1057. doi: 10.1128/jb.136.3.1050-1057.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Spears C., Greene R. C., Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B 12 and methionine. Arch Biochem Biophys. 1972 May;150(1):23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., De Veaux L. C., Mann B. J., Kadner R. J. Separate regulatory systems for the repression of metE and btuB by vitamin B12 in Escherichia coli. Mol Gen Genet. 1987 Mar;206(3):401–407. doi: 10.1007/BF00428878. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Earhart C. F. Gene envY of Escherichia coli K-12 affects thermoregulation of major porin expression. J Bacteriol. 1984 Jan;157(1):262–268. doi: 10.1128/jb.157.1.262-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Margolin W., Krueger J. H., Walker G. C. Mutations affecting regulation of methionine biosynthetic genes isolated by use of met-lac fusions. J Bacteriol. 1982 Aug;151(2):609–619. doi: 10.1128/jb.151.2.609-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Yanofsky C. High level production and rapid purification of the E. coli trp repressor. Nucleic Acids Res. 1986 Oct 24;14(20):7851–7860. doi: 10.1093/nar/14.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Richet E. Maltotriose is the inducer of the maltose regulon of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3059–3061. doi: 10.1128/jb.169.7.3059-3061.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol. 1976 Jul;95(1):173–176. doi: 10.1099/00221287-95-1-173. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]





