Abstract
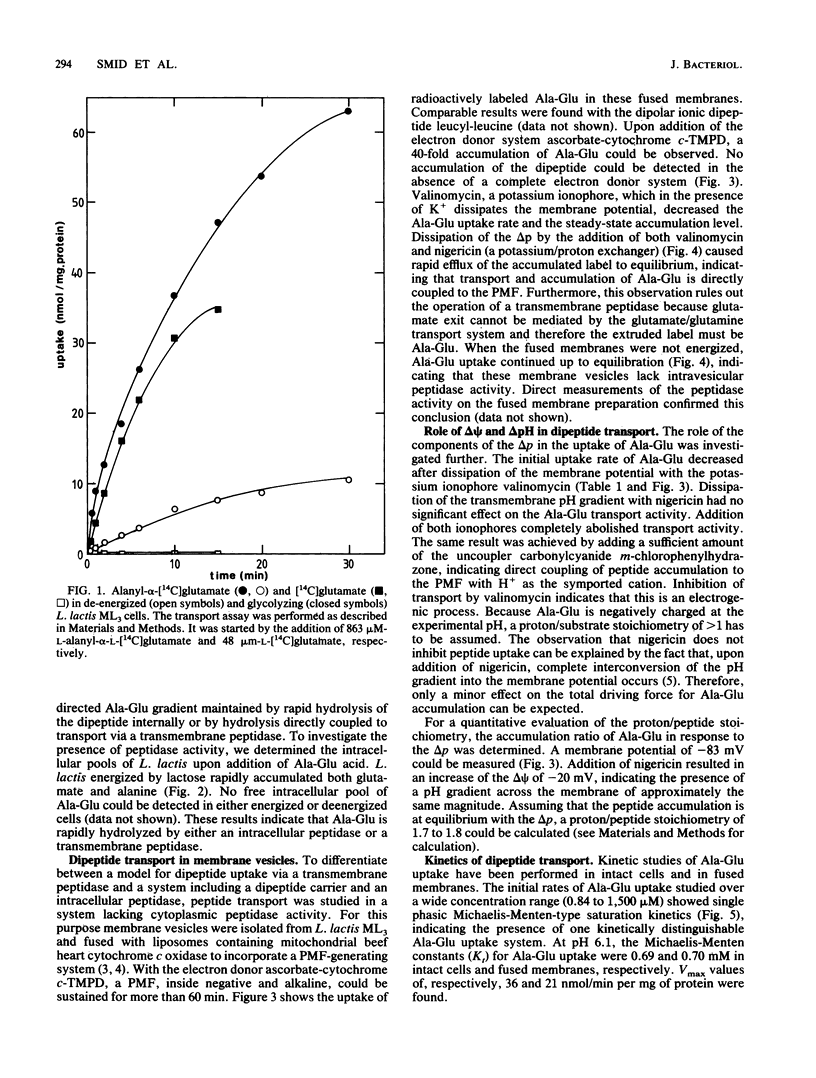
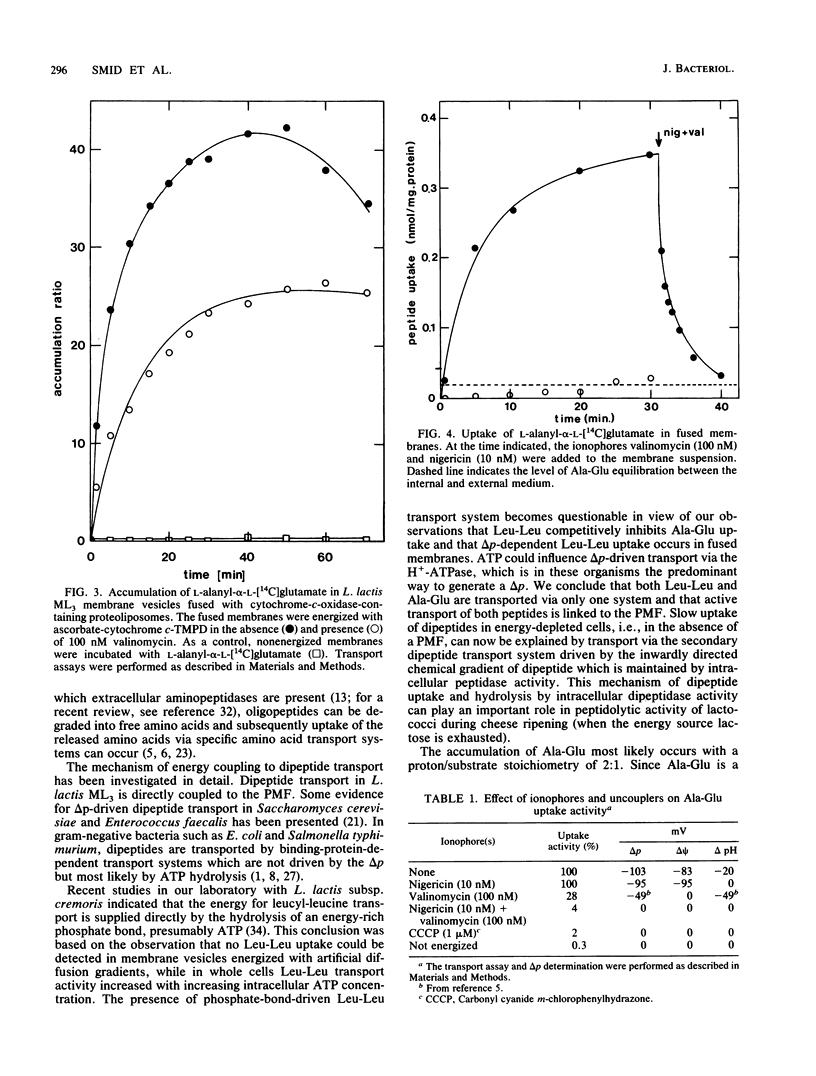
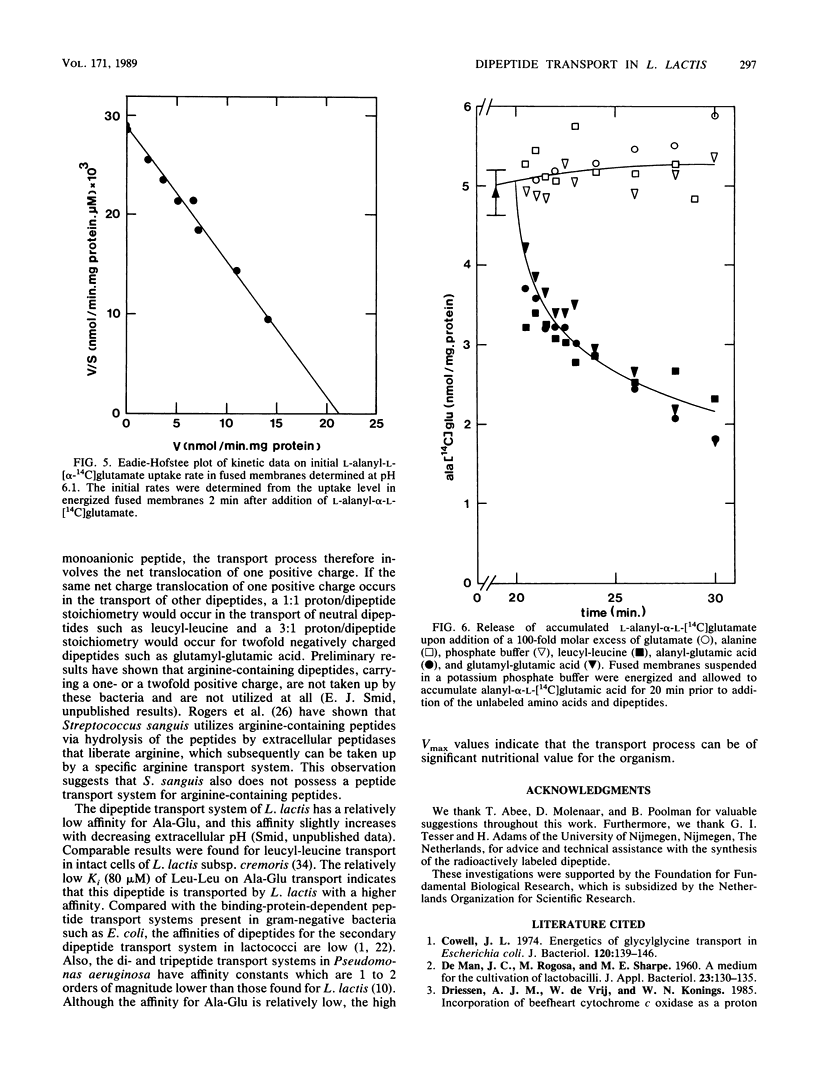
Alanyl-alpha-glutamate transport has been studied in Lactococcus lactis ML3 cells and in membrane vesicles fused with liposomes containing beefheart cytochrome c oxidase as a proton-motive-force-generating system. The uptake of Ala-Glu observed in de-energized cells can be stimulated 26-fold upon addition of lactose. No intracellular dipeptide pool could be detected in intact cells. In fused membranes, a 40-fold accumulation of Ala-Glu was observed in response to a proton motive force. Addition of ionophores and uncouplers resulted in a rapid efflux of the accumulated dipeptide, indicating that Ala-Glu accumulation is directly coupled to the proton motive force as a driving force. Ala-Glu uptake is an electrogenic process and the dipeptide is transported in symport with two protons. In both fused membranes and intact cells the same affinity constant (0.70 mM) for Ala-Glu uptake was found. Accumulated Ala-Glu is exchangeable with externally added alanyl-glutamate, glutamyl-glutamate, and leucyl-leucine, while no exchange occurred upon addition of the amino acid glutamate or alanine. These results indicate that the Ala-Glu transport system has a broad substrate specificity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Poolman B., Kiewiet R., Konings W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6093–6097. doi: 10.1073/pnas.84.17.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gibson M. M. Peptide transport in bacteria. Methods Enzymol. 1986;125:365–377. doi: 10.1016/s0076-6879(86)25031-4. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Kolstad J., Law B. A. Comparative peptide specificity of cell wall, membrane and intracellular peptidases of group N streptococci. J Appl Bacteriol. 1985 May;58(5):449–455. doi: 10.1111/j.1365-2672.1985.tb01484.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law B. A. Peptide utilization by group N streptococci. J Gen Microbiol. 1978 Mar;105(1):113–118. doi: 10.1099/00221287-105-1-113. [DOI] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Nisbet T. M. Limitations to the use of radioactively labelled substrates for studying peptide transport in microorganisms. FEBS Lett. 1980 Sep 22;119(1):73–76. doi: 10.1016/0014-5793(80)81000-3. [DOI] [PubMed] [Google Scholar]
- Perry D., Gilvarg C. Spectrophotometric determination of affinities of peptides for their transport systems in Escherichia coli. J Bacteriol. 1984 Dec;160(3):943–948. doi: 10.1128/jb.160.3.943-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Zilm P. S., Gully N. J., Pfennig A. L. Response of a Streptococcus sanguis strain to arginine-containing peptides. Infect Immun. 1988 Mar;56(3):687–692. doi: 10.1128/iai.56.3.687-692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Tapuhi Y., Schmidt D. E., Lindner W., Karger B. L. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem. 1981 Jul 15;115(1):123–129. doi: 10.1016/0003-2697(81)90534-0. [DOI] [PubMed] [Google Scholar]
- Wiedmeier V. T., Porterfield S. P., Hendrich C. E. Quantitation of Dns-amino acids from body tissues and fluids using high-performance liquid chromatography. J Chromatogr. 1982 Sep 10;231(2):410–417. doi: 10.1016/s0378-4347(00)81865-4. [DOI] [PubMed] [Google Scholar]
- ten Brink B., Otto R., Hansen U. P., Konings W. N. Energy recycling by lactate efflux in growing and nongrowing cells of Streptococcus cremoris. J Bacteriol. 1985 Apr;162(1):383–390. doi: 10.1128/jb.162.1.383-390.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boven A., Konings W. N. A Phosphate-Bond-Driven Dipeptide Transport System in Streptococcus cremoris Is Regulated by the Internal pH. Appl Environ Microbiol. 1987 Dec;53(12):2897–2902. doi: 10.1128/aem.53.12.2897-2902.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boven A., Konings W. N. Energetics of Leucyl-Leucine Hydrolysis in Streptococcus cremoris Wg(2). Appl Environ Microbiol. 1986 Jan;51(1):95–100. doi: 10.1128/aem.51.1.95-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


