Abstract
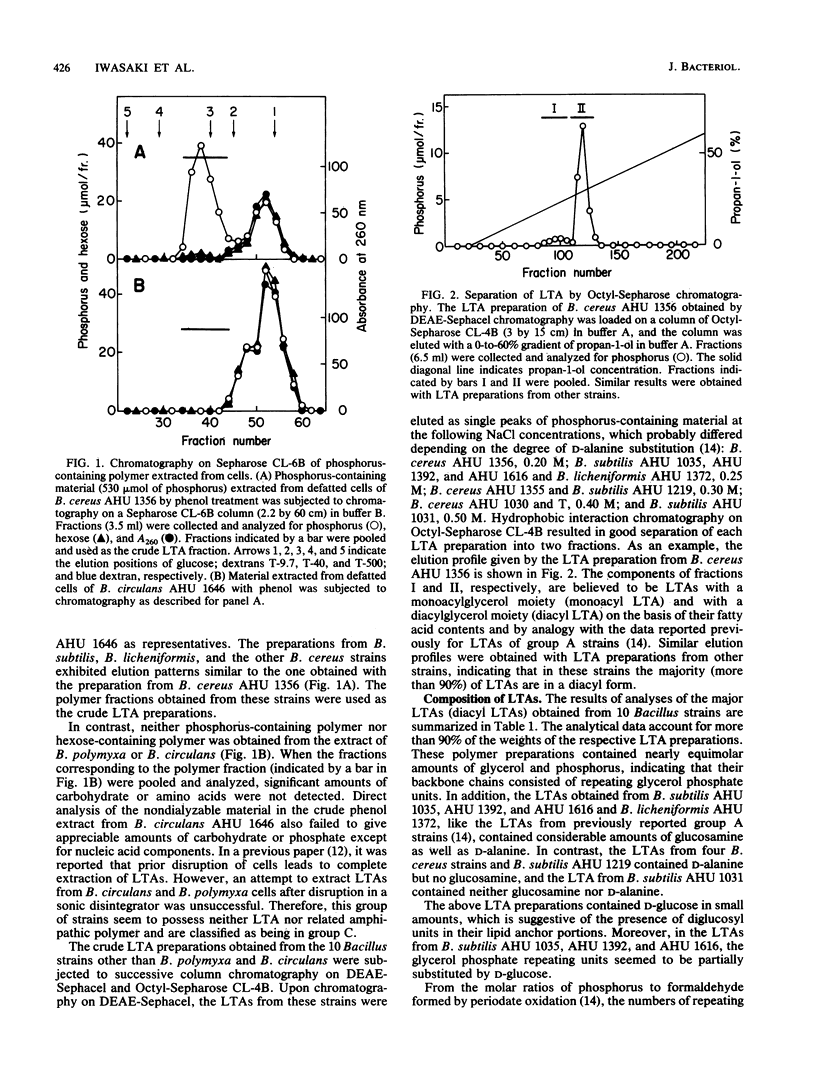
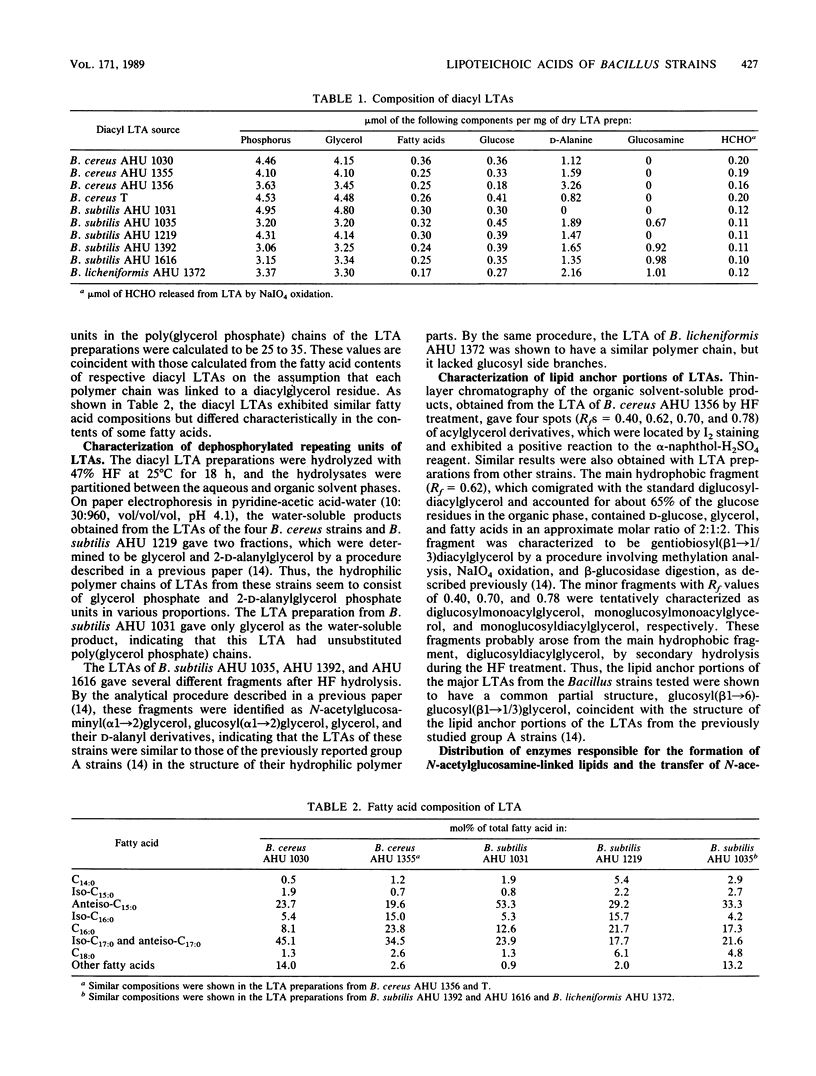
The occurrence, structure, and glycosylation of lipoteichoic acids were studied in 15 Bacillus strains, including Bacillus cereus (4 strains), Bacillus subtilis (5 strains), Bacillus licheniformis (1 strain), Bacillus polymyxa (2 strains), and Bacillus circulans (3 strains). Whereas in the cells of B. polymyxa and B. circulans neither lipoteichoic acid nor related amphipathic polymer could be detected, the cells of other Bacillus strains were shown to contain lipoteichoic acids built up of poly(glycerol phosphate) backbone chains and hydrophobic anchors [gentiobiosyl(beta 1----1/3)diacylglycerol or monoacylglycerol]. The lipoteichoic acid chains of the B. licheniformis strain and three of the B. subtilis strains had N-acetylglucosamine side branches, but those of the B. cereus strains and the remaining two B. subtilis strains did not. The membranes of the B. licheniformis strain and the first three B. subtilis strains exhibited enzyme activities for the synthesis of beta-N-acetylglucosamine-P-polyprenol and for the transfer of N-acetylglucosamine from this glycolipid to endogenous acceptors presumed to be lipoteichoic acid precursors. In contrast, the membranes of the other strains lacked both or either of these two enzyme activities. The correlation between the occurrence of N-acetylglucosamine-linked lipoteichoic acids and the distribution of these enzymes is consistent with the previously proposed function of beta-N-acetylglucosamine-P-polyprenol as a glycosyl donor in the introduction of alpha-N-acetylglucosamine branches to lipoteichoic acid backbone chains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Hazama S., Araki Y., Ito E. Isolation and characterization of structural components of Bacillus cereus AHU 1356 cell walls. Eur J Biochem. 1977 May 16;75(2):513–522. doi: 10.1111/j.1432-1033.1977.tb11552.x. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Ito E. Biosynthesis of N-acetylmannosaminuronic-acid-containing cell-wall polysaccharide of Bacillus subtilis. Eur J Biochem. 1984 Sep 17;143(3):635–642. doi: 10.1111/j.1432-1033.1984.tb08416.x. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Ito E. Biosynthetic studies on N-acetylmannosaminuronic acid containing teichuronic acid in Bacillus megaterium. Can J Microbiol. 1986 Oct;32(10):822–825. doi: 10.1139/m86-151. [DOI] [PubMed] [Google Scholar]
- Arakawa H., Shimada A., Ishimoto N., Ito E. Occurrence of ribitol-containing lipoteichoic acid in Staphylococcus aureus H and its glycosylation. J Biochem. 1981 May;89(5):1555–1563. doi: 10.1093/oxfordjournals.jbchem.a133349. [DOI] [PubMed] [Google Scholar]
- Bok S. H., Demain A. L. An improved colorimetric assay for polyols. Anal Biochem. 1977 Jul;81(1):18–20. doi: 10.1016/0003-2697(77)90593-0. [DOI] [PubMed] [Google Scholar]
- Enghofer E., Kress H. An evaluation of the Morgan--Elson assay for 2-amino-2-deoxy sugars. Carbohydr Res. 1979 Nov;76:233–238. doi: 10.1016/0008-6215(79)80022-1. [DOI] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W. 'Lipoteichoic acid' of Bifidobacterium bifidum subspecies pennsylvanicum DSM 20239. A lipoglycan with monoglycerophosphate side chains. Eur J Biochem. 1987 Jun 15;165(3):639–646. doi: 10.1111/j.1432-1033.1987.tb11488.x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W., Rösel P., Koch H. U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981 May;146(2):467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E. Lipoteichoic acid, a major amphiphile of Gram-positive bacteria that is not readily extractable. J Bacteriol. 1982 Jan;149(1):399–402. doi: 10.1128/jb.149.1.399-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. H., Hancock I. C., Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973 Jan;132(1):83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Shimada A., Ito E. Comparative studies of lipoteichoic acids from several Bacillus strains. J Bacteriol. 1986 Aug;167(2):508–516. doi: 10.1128/jb.167.2.508-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabasakalian P., Kalliney S., Westcott A. Enzymatic blood glucose determination by colorimetry of N,N-diethylaniline-4-aminoantipyrine. Clin Chem. 1974 May;20(5):606–607. [PubMed] [Google Scholar]
- Kawamura T., Kimura M., Yamamori S., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosamine. J Biol Chem. 1978 May 25;253(10):3595–3601. [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Murazumi N., Araki Y., Ito E. Biosynthesis of the wall neutral polysaccharide in Bacillus cereus AHU 1356. Eur J Biochem. 1986 Nov 17;161(1):51–59. doi: 10.1111/j.1432-1033.1986.tb10123.x. [DOI] [PubMed] [Google Scholar]
- Murazumi N., Sasaki Y., Okada J., Araki Y., Ito E. Biosynthesis of glycerol teichoic acid in Bacillus cereus: formation of linkage unit disaccharide on a lipid intermediate. Biochem Biophys Res Commun. 1981 Mar 31;99(2):504–510. doi: 10.1016/0006-291x(81)91773-3. [DOI] [PubMed] [Google Scholar]
- Murazumi N., Yamamori S., Araki Y., Ito E. Anomeric configuration of N-acetylglucosaminyl phosphorylundecaprenols formed in Bacillus cereus Membranes. J Biol Chem. 1979 Dec 10;254(23):11791–11793. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Powell D. A., Duckworth M., Baddiley J. A membrane-associated lipomannan in micrococci. Biochem J. 1975 Nov;151(2):387–397. doi: 10.1042/bj1510387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A., Ohta M., Iwasaki H., Ito E. The function of beta-N-acetyl-D-glucosaminyl monophosphorylundecaprenol in biosynthesis of lipoteichoic acids in a group of Bacillus strains. Eur J Biochem. 1988 Oct 1;176(3):559–565. doi: 10.1111/j.1432-1033.1988.tb14314.x. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Broady K. W., Evans J. D., Knox K. W. New cellular and extracellular amphipathic antigen from Actinomyces viscosus NY1. Infect Immun. 1978 Nov;22(2):615–616. doi: 10.1128/iai.22.2.615-616.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Yamamori S., Murazumi N., Araki Y., Ito E. Formation and function of N-acetyloglucosamine-linked phosphoryl- and pyrophosphorylundecaprenols in membranes from Bacillus cereus. J Biol Chem. 1978 Sep 25;253(18):6516–6522. [PubMed] [Google Scholar]
- Yamamoto T., Koga T., Mizuno J., Hamada S. Chemical and immunological characterization of a novel amphipathic antigen from biotype B Streptococcus sanguis. J Gen Microbiol. 1985 Aug;131(8):1981–1988. doi: 10.1099/00221287-131-8-1981. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Araki Y., Ito E. Biosynthesis of poly(galactosylglycerol phosphate) in Bacillus coagulans. Eur J Biochem. 1987 May 15;165(1):47–53. doi: 10.1111/j.1432-1033.1987.tb11192.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Miyashita T., Araki Y., Ito E. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur J Biochem. 1986 Dec 1;161(2):479–489. doi: 10.1111/j.1432-1033.1986.tb10469.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama T., Koike Y., Arakawa H., Yokoyama K., Sasaki Y., Kawamura T., Araki Y., Ito E., Takao S. Distribution of mannosamine and mannosaminuronic acid among cell walls of Bacillus species. J Bacteriol. 1982 Jan;149(1):15–21. doi: 10.1128/jb.149.1.15-21.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


