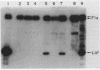
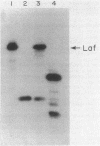
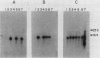
Abstract
Vibrio parahaemolyticus has two distinct cell types, the swimmer cell and the swarmer cell, adapted for locomotion in different circumstances. The swimmer cell, produced when the bacterium is grown in liquid media, is a short rod with a single sheathed polar flagellum. The swarmer cell, produced when V. parahaemolyticus is grown on solidified media, is greatly elongated and synthesizes, in addition to the polar flagellum, numerous unsheathed lateral flagella which are responsible for translocation over surfaces. We are interested in understanding how this bacterium differentiates in response to contact with surfaces and have determined in earlier work that the polar flagellum acts as a tactile sensor which controls transcription of genes (laf) encoding the swarmer cell phenotype. Surface recognition involves sensing of forces that obstruct movement of the polar flagellum. In this report we show that a second signal, iron limitation, is also required for swarmer cell differentiation. Production of lateral flagella occurred only when polar flagellar function was perturbed and iron-limiting growth conditions were imposed. The same conditions were required to induce light production in strains of V. parahaemolyticus in which a laf gene was transcriptionally fused to the lux operon encoding the enzymes for bioluminescence. The lafA gene encoding the lateral flagellin subunit was cloned and used in Northern (RNA) blot measurements. Examination of mRNA levels revealed that transcription of lafA is dependent on growth in iron-depleted media. The control of differentiation by multiple environmental stimuli is discussed.
Full text
PDF

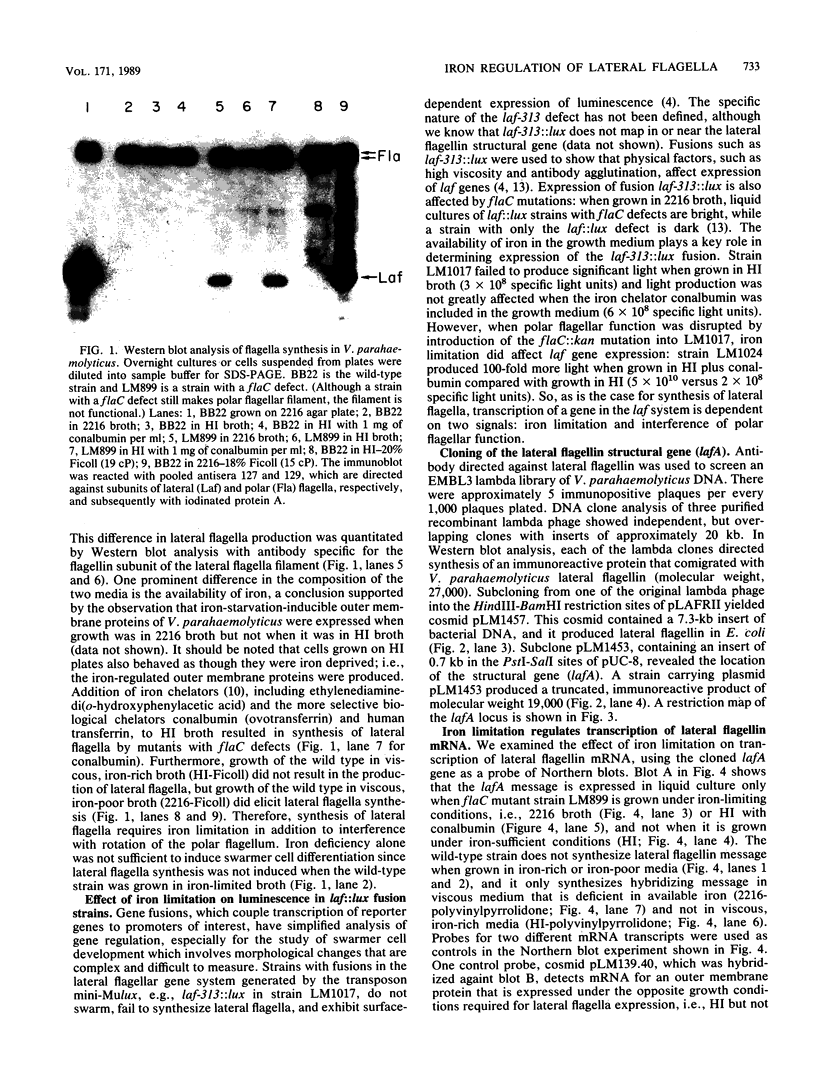



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971 Jul;107(1):295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Belas R., Mileham A., Simon M., Silverman M. Transposon mutagenesis of marine Vibrio spp. J Bacteriol. 1984 Jun;158(3):890–896. doi: 10.1128/jb.158.3.890-896.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972 Dec;36(4):478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarter L. L., Silverman M. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J Bacteriol. 1987 Aug;169(8):3441–3449. doi: 10.1128/jb.169.8.3441-3449.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L., Hilmen M., Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988 Jul 29;54(3):345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Adams D. A. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:61–87. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Honda T., Takeda Y., Miwatani T. Antigenic difference between polar montrichous and peritrichous flagella of Vibrio parahaemolyticus. J Bacteriol. 1974 Nov;120(2):923–928. doi: 10.1128/jb.120.2.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Okamoto K. Formation and function of Vibrio parahaemolyticus lateral flagella. J Bacteriol. 1977 Mar;129(3):1266–1271. doi: 10.1128/jb.129.3.1266-1271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S. Induction of swarming in Vibrio parahaemolyticus. Arch Microbiol. 1974;101(4):357–363. doi: 10.1007/BF00455952. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]