Abstract
Biosynthesis of cephalosporin antibiotics involves an expansion of the five-membered thiazolidine ring of penicillin N to the six-membered dihydrothiazine ring of deacetoxycephalosporin C by a deacetoxycephalosporin C synthetase (DAOCS) enzyme activity. Hydroxylation of deacetoxycephalosporin C to form deacetylcephalosporin C by a deacetylcephalosporin C synthetase (DACS) activity is the next step in biosynthesis of cephalosporins. In Cephalosporium acremonium, both of these catalytic activities are exhibited by a bifunctional enzyme, DAOCS-DACS, encoded by a single gene, cefEF. In Streptomyces clavuligerus, separable enzymes, DAOCS (expandase) and DACS (hydroxylase), catalyze these respective reactions. We have cloned, sequenced, and expressed in E. coli an S. clavuligerus gene, designated cefE, which encodes DAOCS but not DACS. The deduced amino acid sequence of DAOCS from S. clavuligerus (calculated Mr of 34,519) shows marked similarity (approximately 57%) to the deduced sequence of DAOCS-DACS from C. acremonium; however, the latter sequence is longer by 21 amino acid residues.
Full text
PDF
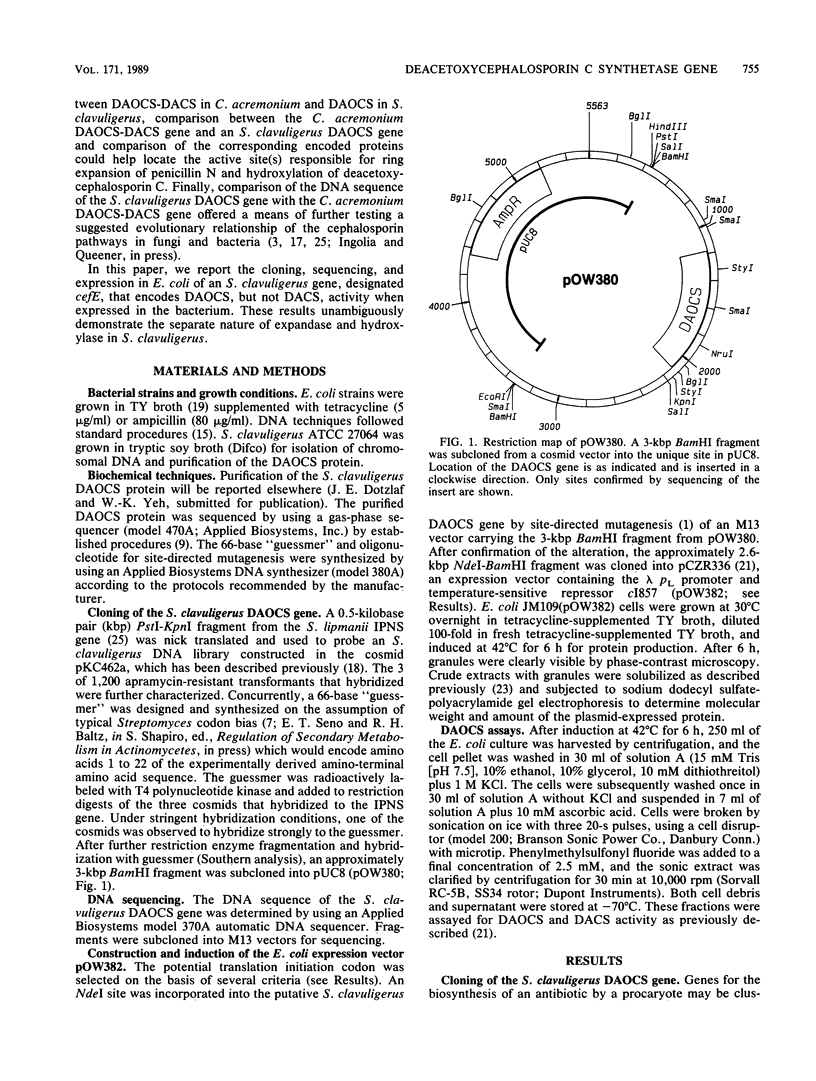


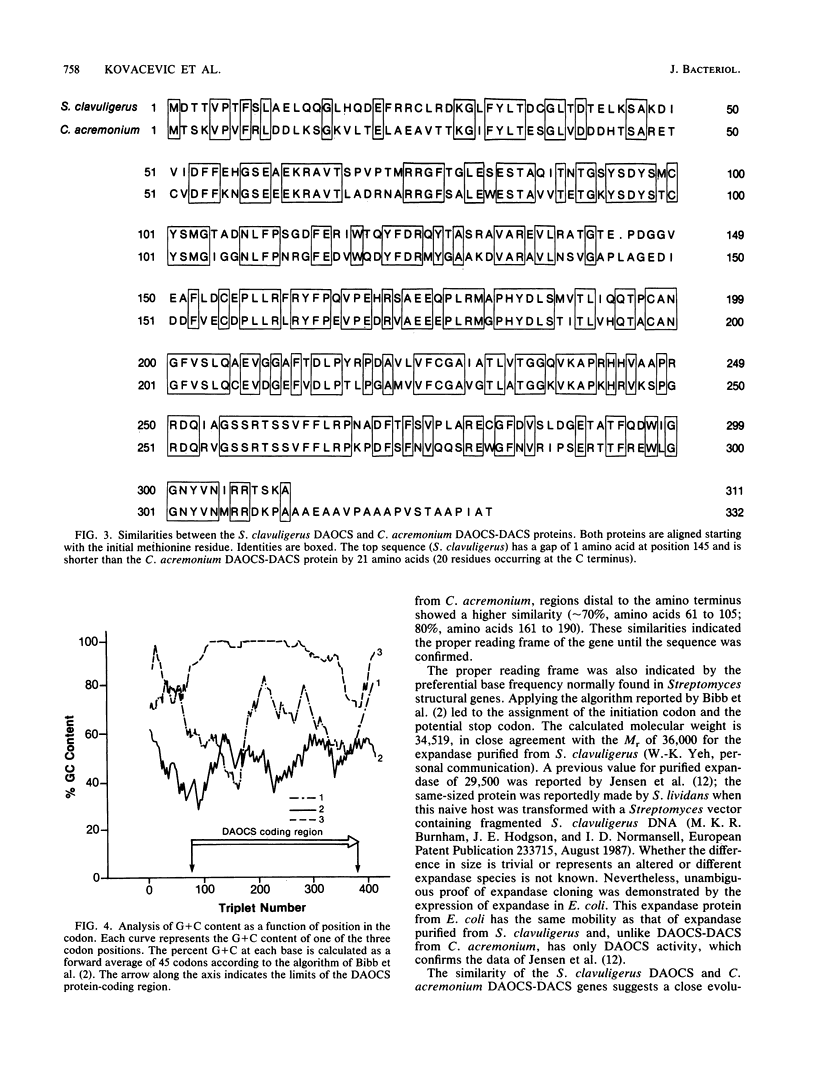
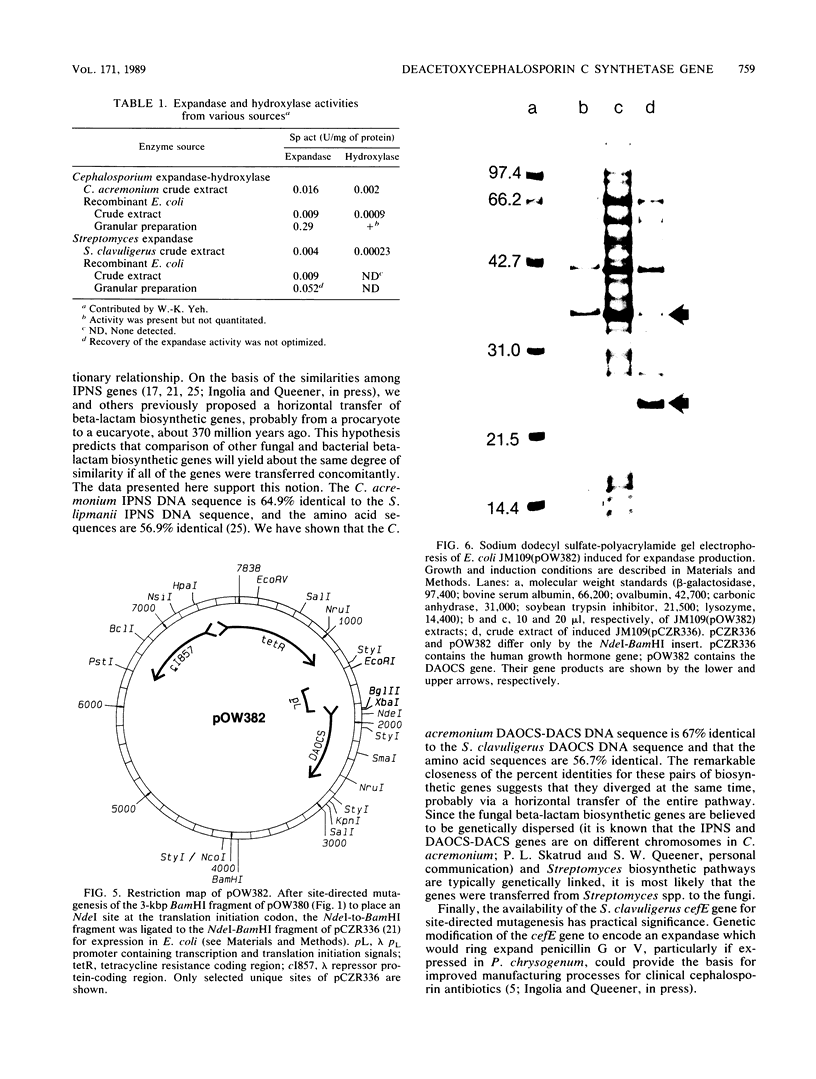

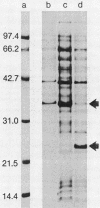
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Hayflick J. S., Vasser M., Seeburg P. H. In vitro deletional mutagenesis for bacterial production of the 20,000-dalton form of human pituitary growth hormone. DNA. 1983;2(3):183–193. doi: 10.1089/dna.1983.2.183. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Cortés J., Martín J. F., Castro J. M., Láiz L., Liras P. Purification and characterization of a 2-oxoglutarate-linked ATP-independent deacetoxycephalosporin C synthase of Streptomyces lactamdurans. J Gen Microbiol. 1987 Nov;133(11):3165–3174. doi: 10.1099/00221287-133-11-3165. [DOI] [PubMed] [Google Scholar]
- Dotzlaf J. E., Yeh W. K. Copurification and characterization of deacetoxycephalosporin C synthetase/hydroxylase from Cephalosporium acremonium. J Bacteriol. 1987 Apr;169(4):1611–1618. doi: 10.1128/jb.169.4.1611-1618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Cox K., Larson J. L., Reynolds P. A., Seno E. T., Yeh W. K., Van Frank R., Hershberger C. L. Cloning genes for the biosynthesis of a macrolide antibiotic. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8248–8252. doi: 10.1073/pnas.84.23.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hollander I. J., Shen Y. Q., Heim J., Demain A. L., Wolfe S. A pure enzyme catalyzing penicillin biosynthesis. Science. 1984 May 11;224(4649):610–612. doi: 10.1126/science.6546810. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Deacetoxycephalosporin C synthetase and deacetoxycephalosporin C hydroxylase are two separate enzymes in Streptomyces clavuligerus. J Antibiot (Tokyo) 1985 Feb;38(2):263–265. doi: 10.7164/antibiotics.38.263. [DOI] [PubMed] [Google Scholar]
- Leskiw B. K., Aharonowitz Y., Mevarech M., Wolfe S., Vining L. C., Westlake D. W., Jensen S. E. Cloning and nucleotide sequence determination of the isopenicillin N synthetase gene from Streptomyces clavuligerus. Gene. 1988;62(2):187–196. doi: 10.1016/0378-1119(88)90557-4. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Pang C. P., Chakravarti B., Adlington R. M., Ting H. H., White R. L., Jayatilake G. S., Baldwin J. E., Abraham E. P. Purification of isopenicillin N synthetase. Biochem J. 1984 Sep 15;222(3):789–795. doi: 10.1042/bj2220789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón D., Carramolino L., Patiño C., Sánchez F., Peñalva M. A. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the beta-lactam ring in Aspergillus nidulans. Gene. 1987;57(2-3):171–181. doi: 10.1016/0378-1119(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Richardson M. A., Kuhstoss S. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 1987;153:166–198. doi: 10.1016/0076-6879(87)53053-1. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Chapman J. L., Belagaje R., Queener S. W., Ingolia T. D. Analysis of the role of cysteine residues in isopenicillin N synthetase activity by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5705–5709. doi: 10.1073/pnas.84.16.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger A., Küenzi M. T., Nüesch J. Partial purification and catalytic properties of a bifunctional enzyme in the biosynthetic pathway of beta-lactams in Cephalosporium acremonium. J Antibiot (Tokyo) 1984 May;37(5):522–531. doi: 10.7164/antibiotics.37.522. [DOI] [PubMed] [Google Scholar]
- Schoner B. E., Belagaje R. M., Schoner R. G. Translation of a synthetic two-cistron mRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8506–8510. doi: 10.1073/pnas.83.22.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel B. J., Burgett S. G., Chen V. J., Skatrud P. L., Frolik C. A., Queener S. W., Ingolia T. D. Cloning and expression in Escherichia coli of isopenicillin N synthetase genes from Streptomyces lipmanii and Aspergillus nidulans. J Bacteriol. 1988 Sep;170(9):3817–3826. doi: 10.1128/jb.170.9.3817-3826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]