Abstract
Central nervous system glial cells release and respond to nucleotides under both physiological and pathological conditions, suggesting that these molecules play key roles in both normal brain function and in repair after damage. In particular, ATP released from astrocytes activates P2 receptors on astrocytes and other brain cells, allowing a form of homotypic and heterotypic signalling, which also involves microglia, neurons and oligodendrocytes. Multiple P2X and P2Y receptors are expressed by both astrocytes and microglia; however, these receptors are differentially recruited by nucleotides, depending upon specific pathophysiological conditions, and also mediate the long-term trophic changes of these cells during inflammatory gliosis. In astrocytes, P2-receptor-induced gliosis occurs via activation of the extracellular-regulated kinases (ERK) and protein kinase B/Akt pathways and involves induction of inflammatory and anti-inflammatory genes, cyclins, adhesion and antiapoptotic molecules. While astrocytic P2Y1 and P2Y2,4 are primarily involved in short-term calcium-dependent signalling, multiple P2 receptor subtypes seem to cooperate to astrocytic long-term changes. Conversely, in microglia, exposure to inflammatory and immunological stimuli results in differential functional changes of distinct P2 receptors, suggesting highly specific roles in acquisition of the activated phenotype. We believe that nucleotide-induced activation of astrocytes and microglia may originally start as a defence mechanism to protect neurons from cytotoxic and ischaemic insults; dysregulation of this process in chronic inflammatory diseases eventually results in neuronal cell damage and loss. On this basis, full elucidation of the specific roles of P2 receptors in these cells may help exploit the beneficial neuroprotective features of activated glia while attenuating their harmful properties and thus provide the basis for novel neuroprotective strategies that specifically target the purinergic system.
Key words: adenine nucleotides, astrocytes, calcium-mediated communication, microglia, neuroprotection, oligodendroglia, P2 receptors, reactive gliosis, sugar nucleotides, uracil nucleotides
Introduction
The roles of extracellular nucleotides as signalling molecules are now well established. ATP (and maybe other nucleotides) is co-localised with “classical” neurotransmitters at many central and peripheral synapses, from which it can be released upon presynaptic depolarising stimuli via a regulated secretion pathway (for review, see [1]). Released nucleotides activate specific membrane receptors, which mediate highly specific and diversified functions in many organs and systems: the seven ligand-gated P2X1–7 receptor channels and the eight G-protein-coupled 7-transmembrane spanning P2Y receptors (P2Y1,2,4,6,11,12,13,14 receptor subtypes) [2–4].
In recent years, evidence has been accumulating to suggest that nucleotides are also released from nonneuronal cells (e.g., circulating platelets and erythrocytes, inflammatory cells, exercising muscle cells, hypoxic cardiomyocytes) and, within the central nervous system, from glial cells. It was initially believed that release of nucleotides from glia could only occur under pathological conditions (e.g., brain ischaemia and trauma) as a consequence of leakage from the cytosolic nucleotide pool due to loss of membrane permeability under milder stress or hypoxic conditions, or as a result of nucleic acid degradation upon cell death due to persistent or stronger insults. It is now known that significant nucleotide release from glia (astroglia, in particular) can also occur under physiological conditions, suggesting that these molecules not only participate in brain repair and recovery after damage but may also play key roles in normal brain function (see also below).
As mentioned above, the highly specific functions of nucleotides are mediated by the activation of P2X and P2Y receptors on target cells. While P2X receptors are believed to mainly respond to ATP and ADP, the P2Y receptor family currently encompasses (1) adenine nucleotide-responding receptors (P2Y1, P2Y11, P2Y12 and P2Y13 subtypes), (2) uracil nucleotide-preferring receptors responding to either UTP or UDP (human P2Y4, P2Y6), (3) receptors of mixed selectivity responding to both ATP and UTP (P2Y2, rodent P2Y4), and (4) the recently recognised P2Y14 receptor, which exclusively responds to sugar nucleotides [4]. P2X receptors are channels for sodium and calcium and may occur as homo- or heterooligomers of more than one subunit characterised by different pharmacological profiles (see [3]). P2X7 subunits do not hetero-oligomerise (see [3]), and are unique in mediating apoptosis and necrosis of several cell types, including glial cells and possibly neurons. P2Y receptors are coupled to several different transduction pathways, including activation of phospholipase C (PLC), inositol-phosphate formation and release of calcium from intracellular stores in the case of P2Y1,2,4,6,11 receptors [4] and inhibition of cAMP formation in the case of P2Y12,13,14. Additional transduction mechanisms have been recently reported (e.g., [5]).
Both P2X and P2Y receptors are expressed on all types of brain cells, including neurons, astrocytes, microglial cells and oligodendroglia. In neurons, the P2X2, the P2X4/P2X6 and P2Y1 receptors appear to be the predominant receptor types [1]. While P2X receptors mediate fast synaptic responses, P2Y receptors have been proposed to mediate slow changes of neuronal membrane potential in response to non-synaptically released ATP. Localisation of these receptors may be at the axon terminals (presynaptic) or the somato-dendritic region (postsynaptic). While presynaptic P2 receptors may be either excitatory (P2X) or inhibitory (P2Y), postsynaptic P2 receptors appear to be without exception excitatory. For more details on the roles of neuronal P2 receptors, the reader is referred to recent authoritative reviews in the field (e.g., [1, 6]). Here, we aim at critically revising the literature on the presence and roles of P2 receptors in glial cells, with special focus on astroglia. We will, however, briefly summarise the most important findings on P2 receptors in oligodendrocytes and Schwann cells and microglia.
Functional roles of P2 receptors in oligodendrocytes and Schwann cells
Oligodendrocytes and Schwann cells are the myelinforming cells of the central (CNS) and peripheral (PNS) nervous systems, respectively, that wrap layers of myelin membrane around axons to insulate them for impulse conduction. Early studies by Fields and Stevens suggested that action-potential firing in axons can regulate maturation of surrounding immature oligodendroglia and Schwann cells and that purinergic signalling, mediated by the activity-dependent axonal release of ATP and subsequent hydrolysis to adenosine, is one of the principal mechanisms of activity-dependent communication between axons and myelinating glia [7, 8]. In a more recent study, Agresti and coworkers characterised the expression of P2 receptors and their functional activity in rat oligodendrocyte progenitors (OPs) [9]. They found that these cells express different types of P2 receptors and that nucleotide-induced Ca2+ raises are mainly due to the activation of P2X7 ionotropic and ADP-sensitive P2Y1 metabotropic receptors. They also showed that ATP and ADP stimulate the migration and differentiation of these progenitor cells and inhibit their mitogenic response to platelet-derived growth factor, thus confirming an important regulatory role for the adeninenucleotide-preferring P2Y1 receptor subtype.
P2 receptors may also influence oligodendrocyte function at later developmental stages. Addition of ATP analogs to already differentiated oligodendrocytes in coculture with dorsal root ganglion cells produced significantly more myelinated axons [10]. Experiments with more selective P2 receptor agonists showed that the P2Y receptor agonist UTP (300 µM) was ineffective (90.1% ± 13.9% relative to control) whereas the P2X receptor agonist alpha-beta-methylene-ATP (α,βmeATP) (300 µM) increased myelination (169.4% ± 37.0%). Considerable additional research will be required to fully identify the specific P2 receptor subtypes responsible for this effect, but these initial findings do not provide support for involvement of the P2X7, P2Y2, or P2Y4 receptor subtypes in increased myelination.
Recently, a link between electrical impulse activity in axons, astrocytes and myelination that is mediated by the cytokine leukaemia inhibitory factor (LIF) has been demonstrated. In particular, LIF is released by astrocytes in response to ATP liberated from axons firing action potentials (see also Figure 2), and LIF promotes myelination by mature oligodendrocytes [10]. These results reveal a new mechanism by which action potentials influence myelination that involves unexpected interactions between astrocytes and myelinating glia and between purinergic and cytokine signalling.
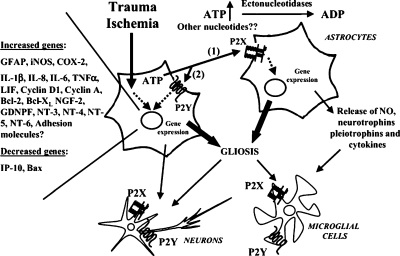
Figure 2.
Modulation of brain inflammation by extracellular nucleotides. Traumatic and/or ischaemic events lead to the massive release of astrocytic ATP, which is rapidly degraded to ADP and maybe other nucleotides. Extracellular nucleotides activate P2Y and P2X receptors either paracrinally (1) or autocrinally (2), leading to modulation of gene expression. Several pro-inflammatory and anti-apoptotic genes have been demonstrated to be specifically upor down-regulated, which in turn results in astrocytic proliferation and growth (reactive astrogliosis). Reactive astrocytes also synthesise and release neurotrophins, pleiotrophins, cytokines, and small signalling molecules [e.g., nitric oxide (NO)], which can modulate neuronal and microglial cell survival and their reaction to noxious stimuli. Extracellular nucleotides can also directly activate ionotropic or metabotropic receptors expressed by cell types other than astrocytes (e.g., neurons and microglia), thus further contributing to the development of a first inflammatory line of defence against spreading of brain damage. Nevertheless, a chronic, sustained, and out-of-control inflammatory reaction may result in brain cell loss, and development of neurodegenerative events (see text for more details). GDNPF glial-derived neurite-promoting factor, IP-10 interferon-inducible protein 10, LIF, leukaemia inhibitory factor NT neurotrophin
Globally, these findings suggest that ATP (and maybe other nucleotides), which is released in high amounts under inflammatory conditions and following cell death (see also below), might regulate remyelination processes in inflammatory demyelinating diseases of the CNS, such as multiple sclerosis.
Functional roles of P2 receptors in microglia
Microglial cells, which react to almost any type of pathological conditions, are thought to play a major role in the immune response that occurs in the CNS. Upon activation, microglial cells acquire features of cytotoxic and phagocytic cells, therefore taking part in the remodelling of the nervous system tissue following insults. Among various substances, including growth factors, cytokines, chemoattractants and neurotransmitters [11], extracellular ATP has been indicated as a key messenger in microglial activation. Functional responses to nucleotides have been reported in microglial cells both in culture [12–14] and in situ [15]. The effects induced by ATP in microglial cells are complex (for review, see [16]). As first analysed in culture, purinergic receptor activation triggers induction of a nonselective cationic and a K+ conductance and leads to an increase in cytosolic [Ca2+] [14]. Pharmacological screening indicates that microglial cells express both P2Y and P2X receptors (see also below). As studied in cell culture, both non-stimulated and stimulated microglial cells express purinergic receptors. In cultured microglia, activation of P2X7 receptors by astrocyte-derived ATP provokes release of interleukin (IL)-1β and also triggers release of plasminogen [12, 17]. Challenge of cultured microglia with lipopolysaccharide (LPS) induced release of tumour necrosis factor alpha (TNFα), IL-6, IL-12 and macrophage inflammatory protein 1α. All these parameters were reduced in the presence of purinergic ligands, suggesting that purinergic receptor activation may attenuate indicators of microglial activation [18]. On the other hand, LPS can per se differentially regulate the responsiveness of P2 receptors (see also below). To determine which subtype(s) of P2 receptors mediate(s) the response of microglia to nucleotides, we recently assessed the presence and activity of P2 receptor subtypes in the mouse microglial N9 cell line. All members of the P2 receptor family were found to be present in these cells at mRNA and/or protein level. The functionality of these receptors was assessed by analysing the calcium responses induced by specific P2X/P2Y agonists. Data suggested that, under resting conditions, a major contribution to [Ca2+]i increases was given by the P2X7 receptor. Among P2Y receptors, P2Y1 and P2Y2/4 play a prominent role, and P2Y6, P2Y12/13 and P2Y14 may also contribute [19]. Importantly, we showed that N9 microglial cells maintain a P2 receptor profile comparable with that of primary microglial cells isolated from rodent embryo, hence validating this cell line as an adequate model to study regulation of microglia by purines. Changes in P2 receptor functionality were induced by exposure of cells to the bacterial endotoxin lipopolysaccharide (LPS), a widely utilised experimental tool to mimic microglia-cell activation in vitro [19]. LPS increased P2Y6 and P2Y14, decreased P2X7 and left P2Y1 and P2Y2,4 receptor activity unchanged. These differential changes suggest selective roles for specific P2 receptor subtypes in the acquisition of the final activated microglia phenotype. Recently, a role for purinergic microglial receptors in tactile allodynia after nerve injury has been suggested [20]. Pharmacological blockade of spinal P2X4 receptors reversed tactile allodynia caused by peripheral nerve injury without affecting acute pain behaviours in naive animals. After nerve injury, expression of P2X4 increased strikingly in hyperactive microglia (but not in neurons or astrocytes) of the ipsilateral spinal cord. Intraspinal administration of P2X4 antisense oligodeoxynucleotide decreased induction of P2X4 and suppressed tactile allodynia after nerve injury. Conversely, intraspinal administration of microglia in which P2X4 had been induced and stimulated produced tactile allodynia in naive rats. Taken together, these findings suggest that microglial P2 receptors may represent novel interesting therapeutic targets for inflammatory neurological diseases characterised by abnormal microglial response, including pain.
Role of extracellular nucleotides and their receptors in astrocytes
Astrocytes play a pivotal role in CNS physiopathology. These cells participate to neuronal migration during brain development [21] and express neurotransmitter transporters and receptors (including P2 receptors, see also below), thus actively participating in neurotransmission [22–24]. Astrocytes also release and respond to chemokines and cytokines [25, 26] and are capable of intense metabolic activity and proliferation (“reactive astrogliosis”) during diverse immune/inflammatory brain diseases (e.g., multiple sclerosis, Alzheimer’s dementia, tumours and neurodegenerative and prion diseases) (for review, see [27] and references therein). There is hence a growing interest in the characterisation of endogenous factors regulating short- and long-term astroglial cell function under both physiological and pathological conditions.
Involvement of extracellular nucleotides in homotypic and heterotypic short-term calcium-dependent signalling in the brain
In recent years, one of the most exciting developments in the field of purinergic transmission has been the demonstration that ATP is the dominant messenger mediating intercellular communication in the brain and that this occurs via activation of P2 receptors on astrocytes. Several years ago, we and other groups demonstrated that exposure of mammalian brain astrocytes to adenine or uracil nucleotides resulted in concentration-dependent increases of intracellular calcium ([28]; for review, see [29]). This effect was antagonised by non-selective P2 receptor antagonists such as suramin and pyridoxalphosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS), thus confirming the involvement of extracellular P2 receptors [28]. More or less in the same period, seminal work by Guthrie and coworkers [30, 31], Newman and Zahs [32] and Haydon [29] demonstrated that mechanical stimulation of a glial cell evoked a local elevation of intracellular calcium that subsequently propagated to neighbouring cells. These calcium waves were initially thought to spread as a result of gap-junction metabolic coupling via diffusion of inositol-tris-phosphate through neighbouring cells. This mechanism indeed explained short-term signalling, but a diffusible factor was shown to be necessary for long-range calcium signalling, and this factor was later identified as ATP [29] (see Figure 1). These findings were then confirmed by several other groups, and the molecular mechanisms responsible for ATP release from astrocytes were characterised in detail. Seminal work by Coco et al. [33] demonstrated that ATP released from astrocytes could also serve as a signalling molecule for other types of brain cells. Specifically, these authors showed that, in astrocyte-microglia co-cultures, mechanical or neurotransmitter (bradykinin) stimulation resulted in calcium elevation in astrocytes, which was followed immediately afterwards by calcium elevation in neighbouring microglia. This effect was blocked by the ATPdegrading enzyme apyrase and by P2 receptor antagonists, thus confirming that ATP was indeed the diffusible factor involved. Coco et al. [33] also demonstrated that astrocytes could concentrate ATP in vesicles (which were distinct from glutamate-containing vesicles), from which ATP could be released via a regulated secretory pathway even under pseudo-physiological conditions.
Figure 1.
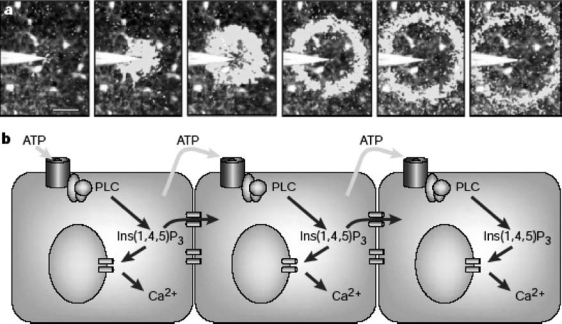
Stimulation of glial cells from the retina evokes a radially propagating wave of elevated calcium. (a) Mechanical stimulation of a glial cell in the centre of the field of view evoked a local elevation of Ca2+. Subsequently, this Ca2+ elevation propagated to neighbouring cells. In this sequence, images were acquired at 0.93-s intervals, and the white overlay image shows the leading edge of the wavefront. (b) Putative mechanism for glial Ca2+ wave generation. Ca2+ is released from internal stores in response to elevated internal inositol-1,4,5-trisphosphate [Ins(1,4,5)P3]. Ins(1,4,5)P3 can diffuse to neighbouring cells through gap junctions to cause short-range signalling. Long-range calcium signalling requires the release of ATP, which causes the regenerative production of Ins(1,4,5)P3 and further release of ATP from neighbouring astrocytes. Reproduced with permission from [29].
The importance of ATP in mediating cell-to-cell communication in the brain has been recently confirmed in vivo in transgenic mice in which all microglia are fluorescently labelled. By utilising time-lapse two-photon imaging of the intact mouse cortex, Davalos et al. [34] showed that the fine termini of microglial processes are highly dynamic. Upon traumatic brain injury, microglial processes rapidly and autonomously converged on the site of injury, establishing a potential barrier between the healthy and injured tissue. This rapid chemotactic response could be mimicked by local injection of ATP and could be inhibited by the ATPhydrolysing enzyme apyrase or by purinergic receptor blockers. The microglial response was also abolished by connexin channel blockers, which are highly expressed in astrocytes and inhibit ATP release from these cells. This represents the first in vivo demonstration that ATP release from brain-damaged tissue and surrounding astrocytes mediates a rapid microglial response towards injury.
Since the first demonstration that ATP could act as signalling molecule in short-term calcium-dependent cell-to-cell communication in the brain, there has been a lot of interest in the characterisation of the specific P2 receptor subtypes involved in this action. In this respect, we characterise the expression and functionality of P2 receptors in rat cortical primary astrocytes [35]. Reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis showed that, with the exception of the P2X6 receptor, these cells express all other P2X (i.e., P2X1–5 and P2X7) and P2Y receptors (i.e., P2Y1, P2Y2, P2Y4, P2Y6, P2Y12 and P2Y14) cloned from rodent tissues (P2Y11 was not investigated based on bioinformatics data, suggesting that this receptor is not present in rat and murine genomes). The P2Y13 receptor (which we originally cloned and characterised from rat tissues in 2004, see [36]) was also found to be expressed at levels comparable to those of the other known P2Y receptors. Thus, rat brain astrocytes surprisingly express a nearly full panel of P2 receptors. Such a large repertoire of P2 receptors is in line with the P2 receptor heterogeneity reported in several other tissues and cells (e.g., [19, 37], suggesting that specific receptor subtypes are differentially recruited and/or inserted into the plasma membrane, depending upon the cell functional state and specific pathophysiological conditions. In line with this hypothesis, single-cell image analysis showed that in rat primary astrocytes, only some of the expressed P2X and P2Y receptors are functionally coupled to increases of [Ca2+]i. While ATP induced rapid and transient [Ca2+]i increases (counteracted by the P2 antagonists suramin, PPADS and oxidised ATP), the P2X1/P2X3 agonist α,βmeATP produced no changes [35]. Conversely, the P2X7 agonist 2′-3′-O-(4-benzoylbenzoyl)-adenosine 5′-triphosphate (BzATP) markedly increased [Ca2+]i and induced the formation of the P2X7 pore, as assayed by ethidium bromide (EtBr) uptake. ADP and 2-methylthio-ATP (2meSATP) also produced [Ca2+]i increases antagonised by the P2Y1 antagonist MRS2179 [35]. Some cells also responded to UTP but not to UDP. Significant responses to the sugar-nucleotides UDP-glucose and UDP-galactose were also detected, which represented the first functional response reported for the P2Y14 receptor in a native system. Based on agonist preference of known P2 receptors, we concluded that in rat cortical astrocytes ATP-induced calcium rises are at least mediated by P2X7 and P2Y1 receptors; additional receptors (i.e., P2X2, P2X4, P2X5, P2Y2, P2Y4 and P2Y14) may also contribute. Our results highlighting a role for P2Y1 and P2Y2 receptors in shortterm calcium signalling are consistent with data from Gallagher and Salter [38], showing that these receptors are both necessary for propagation of calcium waves in rat spinal cord astrocytes and in human 1321N1 astrocytoma cells. These authors also showed that calcium waves propagating through P2Y2 travel faster and further. Interestingly, apyrase accelerated propagation through P2Y1 and blocked propagation through P2Y2, suggesting that the level of expression of nucleotidases may regulate the extent of activation of the two receptors, thus affecting the characteristics of calcium waves. It must be emphasised, however, that these studies have been performed on human astrocytoma cells heterologously expressing these receptor subtypes. Our data on the rat primary astrocytes suggest a role also for the P2X7 receptor, in line with previous findings demonstrating that treatment of rat astrocytic cultures with BzATP increased influx of extracellular Ca2+ and [Ca2+]i [39]. However, no study has investigated the possible role of this receptor subtype in in vivo short-term calcium-dependent signalling.
In conclusion, these findings demonstrate that upon mechanical or neurotransmitter stimuli, brain astrocytes can release and respond to ATP with a propagating wave of intracellular calcium increases, allowing a homotypic, astrocyte-to-astrocyte, communication as well as a heterotypic signalling, which involves adjacent brain cells, including neurons, oligodendrocytes and microglia [40].
Involvement of extracellular nucleotides in long-term morphological and functional changes of astrocytes (reactive astrogliosis)
Brain astrocytes are also known to undergo reactive astrogliosis, a phenomenon characterised by astroglial proliferation, cellular hypertrophy, enhanced synthesis of neurotrophins, pleiotrophins and inflammatory mediators and elongation of glial fibrillary acidic protein (GFAP)-positive astrocytic processes (for review, see [27]). Sustained inflammatory astrogliosis is detected in the mammalian brain following various kinds of traumatic or hypoxic insults (e.g., stroke), as well as chronic neurodegenerative and demyelinating disorders (Alzheimer’s, Parkinson’s, Huntington’s diseases and multiple sclerosis; for reviews, see [27, 41]. Reactive glia seems to function as a double-edged sword for neurons. On one side, reactive astrocytes can impede neuronal survival and regeneration by forming scar tissue and synthesising neurotoxic molecules [including cytokines, nitric oxide (NO) and arachidonic acid (AA) metabolites] [41]. However, the net effect of glia-mediated neuroinflammation is sometimes beneficial [41]. For example, in the PNS, inflammation is necessary for the successful regeneration that is impeded in the CNS, and loss of astrocytes impairs recovery [42]. Moreover, activated astrocytes are needed for axonal growth and guidance, to promote repair, produce energy substrates and neurotrophins, act as free radicals and glutamate scavengers, restore the blood-brain barrier and promote neovascularisation and neurogenesis—i.e., the generation of new neurons that continues throughout life and contributes to brain recovery after damage. Interestingly, while acute insults trigger “compensatory” neurogenesis, chronic neuroinflammation is detrimental to regeneration [43]. Thus, inflammatory gliosis in the brain likely starts as a time- and site-specific defence mechanism that could later evolve as a destructive and uncontrolled reaction, thus contributing to brain damage.
Extracellular nucleotides have been implicated as endogenous triggers of reactive astrogliosis. Upon trauma or ischaemia, brain cells are exposed to elevated concentrations of nucleotides and nucleosides for prolonged periods as a result of leakage from the nucleotide cytosolic pool (due to loss of membrane permeability) or nucleic acid degradation upon cell death due to persistent or stronger insults. Purines and pyrimidines can indeed produce all the hallmark characteristics of astrogliosis both in vitro and in vivo (see Figure 2).
The first evidence in this respect dates back to 1987 when Kim and Rathbone were purifying from brain proteins that stimulated proliferation of cultured chick-brain astrocytes. They isolated a potently mitogenic fraction that was not a protein, and chemical analysis identified it as 5′-GMP. Rathbone and his colleagues soon determined that guanosine, adenosine and their respective 5′-mono-, di- and triphosphate derivatives also stimulated proliferation and DNA synthesis of chick astrocytes in vitro (see [44] and references therein). Simultaneously, Neary, Norenberg and colleagues, observed stellation and increases in GFAP content within 1 h of exposure of rat cerebral cortical astrocytes to 100 µM ATP. After 72-h treatment with 1 mM ATP, DNA synthesis was increased three- to four-fold (see [44] and references therein).
As a follow-up of these initial observations, we determined the ability of the hydrolysis-resistant ATP analog α,βmeATP to influence both proliferation and differentiation of primary rat striatal astrocytes in culture. Exposure to α,β meATP increased the percentage of bromodeoxyuridine-positive cells, suggesting increased proliferation [45]. Moreover, this nucleotide also induced a concentration-dependent increase of GFAP-positive astrocytic processes [46]. Interestingly, the effects induced by the ATP analogue were quantitatively similar to those produced by basic fibroblast growth factor (bFGF), a known trigger of reactive astrogliosis. In fact, both ATP and the polypeptide growth factor promoted maturation of astrocytes towards a more differentiated phenotype characterised by longer and thicker astrocytic processes [46]. In subsequent studies, similar effects were obtained with other nucleotides, such as ATP, adenosine 5′-O-(2-thiodiphosphate) (ADPβS), 2meSATP, βγmeATP and UTP. Nucleotide-induced astrogliosis was inhibited by pertussis toxin (suggesting the involvement of a G-protein-coupled P2Y receptor) and by several typical P2 receptor antagonists, such as suramin and PPADS, but not by P2 receptor antagonists that preferentially block P2X receptors, such as 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) [47, 48]. Importantly, the relevance of these in vitro findings to in vivo reactive astrogliosis was confirmed by Franke and coworkers [49] while investigating in the nucleus accumbens of rats the morphological effects induced by local application of P2X and P2Y ligands. A strong mitogenic effect was induced on astrocytes by ADPβS, 2meSATP, α,βmeATP and, to a smaller extent, UTP-γS. This paper was, indeed, the first to confirm that effects induced by extracellular nucleotides in in vitro systems do have a functional significance for in vivo gliosis, thus validating the studies on cultured cells as adequate experimental models to investigate the roles of P2 receptors in reactive astrogliosis.
Transduction pathways involved in membrane-to-nucleus signalling at the basis of nucleotide-induced astrogliosis
In 1997, we observed that at very early times (15 min) after nucleotide challenge, cultured astrocytes released AA, as shown by measurement of radioactivity in the culture medium of cells previously labelled with [3H]-AA [47]. This suggested that activation of phospholipase A2 (PLA2) could be involved in the long-term functional effects evoked by extracellular nucleotides. Consistently, exogenously added AA markedly elongated astrocytic processes [47], as did the inflammatory prostaglandin E2 (the major AA metabolite in the brain) [50]. In line with these findings, various PLA2 inhibitors (e.g., mepacrine and dexamethasone) prevented both the early nucleotide-induced [3H]-AA release and the associated long-term morphological changes [47]. These effects were highly specific for the purinergic system since in the same cells inhibition of PLA2 had no effect on astrocytic elongation induced by classical growth factors such as bFGF [47]. In 1999, we demonstrated that exposure of rat primary astrocytes to NS-398, a selective inhibitor of the inducible mitogen-stimulated cyclooxygenase-2 (COX-2), significantly prevented P2Y receptor-mediated reactive astrogliosis [50]. Subsequent RT-PCR and Western blot analysis showed that nucleotide-induced gliosis is indeed accompanied by the de novo synthesis of COX-2 [50]. COX-2 up-regulation was fully prevented by PPADS, thus confirming a role for P2 receptors. Globally, these data suggest that nucleotides induce astrocytes to release AA, which is then metabolised by COX-2 to PGE2, which, in turn, likely represents the AA product mediating nucleotide-induced reactive astrogliosis.
Due to the large heterogeneity of P2 receptors in astrocytes (see above and [35]), it may well be that activation of the signalling cascade culminating in reactive astrogliosis involves more that one P2 receptor or requires interaction between different receptor subtypes, as also suggested by studies performed in vivo [49].
The receptor-to-nucleus signalling cascade responsible for nucleotide-induced reactive gliosis in rat primary astrocytes has been dissected in detail and represents one of the few examples of characterised gene transcriptional control by P2 receptors. In our experimental model, stimulation of P2Y receptors on astrocytes led to activation of extracellular-regulated kinases 1/2 (ERK1/2) via multiple parallel signalling pathways involving Gi/o protein-dependent and calcium-independent stimulation of phosphatidylcholine phospholipase C (PC-PLC) and/or phospholipase D (PLD), with consequent activation of the Ras/Raf system [51]. Involvement of extracellular nucleotides and ERK1/2 in activation of astrocytes has been also confirmed in another experimental model of gliosis: stretch-induced astrocyte injury. Application of a rapid and reversible stretch-induced injury to cultured astrocytes resulted in immediate ERK1/2 activation, which was already maximal after 10 min [52]. ERK activation was prevented by apyrase and P2 receptor antagonists, suggesting that injury causes release of ATP, which may in turn autocrinally and heterocrinally signal on astrocytes and stimulate ERK1/2 activity. Studies with various P2 receptor antagonists indicated that in this experimental model, activation of P2Y1 and P2X2 receptor subtypes might be particularly important for the detected effects. Besides ERKs, in rat cortical astrocytes, P2X and P2Y agonists may also stimulate Akt/protein kinase B system, another family of protein kinases that has been implicated in cell growth and survival, in angiogenesis and glycogen synthesis [53, 54]. In astrocytes, a number of adenine and uracil nucleotides induced Akt phosphorylation: pertussis toxin inhibited ATP and UTP-(but not BzATP-) induced effects, suggesting a role for P2X7 and P2Y receptors [53, 54]. At present, it is not completely clear if signalling via ERK and Akt may represent two independent pathways of gliosis; however, pharmacological data suggest that not all P2 receptor antagonists that effectively inhibit ERK also inhibit Akt, suggesting involvement of either one of both pathways, depending upon type, strength and length of the insult.
Modulation of gene expression by extracellular nucleotides in reactive gliosis
Both the ERK and the Akt pathways are known to influence gene expression through phosphorylation/activation of transcriptional factors [55]. Evidence has accumulated to suggest induction of specific gene families in glial cells upon exposure to extracellular nucleotides and activation of P2 receptors. One of the first evidences in this respect dates back to 1997, when we reported nuclear accumulation of the fos and jun proteins upon exposure of striatal astrocytes to nucleotides [47]. At that time, these proteins were already known to dimerise and form activated AP-1 transcriptional factors that can modulate gene expression by binding to specific sequences on the promoter regions of many genes. In line with this hypothesis, a few years later, we demonstrated that exposure of cultured astrocytes to nucleotides resulted in a time-dependent increase in DNA binding activity of AP-1 [51]. In the same experiments, extracellular nucleotides also increased DNA binding of nuclear factor kappa B (NF-kB), another transcriptional factor crucially involved in several inflammatory events including reactive astrogliosis. The temporal profile of AP-1- and NF-kB-induced DNA binding, which was significantly increased 30 min after nucleotide addition to cultures and reached a maximal peak at 1 h, was consistent with the timing of ERK1/2 activation, the latter already being maximally activated after a 10-min exposure to purine analogs [51]. Globally, these data demonstrated a causal relationship between ERK1/2 and binding of transcriptional factors to DNA, suggesting a molecular pathway for regulation of gene expression by extracellular nucleotides. Importantly, these data are also consistent with results obtained on human foetal astrocytes, where extracellular nucleotides potentiate IL-1β-induced NF-kB and AP-1 activation, nuclear translocation and DNA binding [56]. Interestingly enough, pharmacological data indicated involvement of distinct P2 receptors in the activation of these two families of transcriptional factors. In particular, ATP and ADP were equipotent in inducing NF-kB activation (followed by BzATP, with no effect of UTP) whereas ADP was the most potent trigger for AP-1 activation (followed by ATP, with no effect by either BzATP or UTP). Besides indicating different roles for different P2 receptors in modulation of IL-1β-mediated signal transduction, these results suggest highly specific effects of extracellular nucleotides on gene transcription (see also below).
More details on the gene families regulated by P2 receptors in astroglial cells have been recently provided by Chorna et al. [57]. These authors showed that in 1321N1 astrocytoma cells expressing P2Y2 receptors, acute treatment with UTP upregulated anti-apoptotic genes Bcl-2 and Bcl-Xl and downregulated the proapoptotic gene Bax. UTP also upregulated a number of genes for neurotrophins, neuropeptides and growth factors (see also Figure 2). This pattern of gene expression clearly indicated activation of neuroprotective mechanisms; this conclusion is further confirmed by the demonstration that cultured media from UTP-treated cells stimulated neurite outgrowth in PC-12 cells. In line with these findings, activation of P2Y6 in human astrocytoma cells prevented TNFα-induced apoptosis and inhibited TNFα-induced activation of caspase-3 and caspase-8 [58]. Although all these results have been obtained in cell lines expressing recombinant P2 receptors, they globally suggest that these receptors may mediate a protective mechanism and that the net effect of purine-induced gliosis may be beneficial. There may even be some novel, yet-uncharacterised neuroprotective purinergic receptors. In fact, besides releasing adenine nucleotides, astrocytes have been shown to release guanine-based purines under basal and hypoxia conditions [59–61]. Guanosine is antiapoptotic for neuroblastoma cells and also protects astrocytes from apoptosis via upregulation of Bcl-2 [61]. The mechanism at the basis of guanosine protective effects are still unknown but activation of a novel guanosinespecific receptor may be involved. Globally, these findings are consistent with results indicating increased ectonucleotidase expression in focal cerebral ischaemia [62]. In particular, data indicated an up-regulation of the capacity for hydrolysis of nucleotides within tissue adjacent to the infarcted area, suggesting that nucleotide concentrations at the site of injury may crucially regulate the onset and development of reactive and repair mechanisms.
The large variety of genes that have been reported to be induced by extracellular nucleotides in astroglial cells is summarised in Figure 2. It is evident that some genes are increased and some other genes are decreased, which suggests a high degree of specificity and a role for P2 receptors in fine-tuning gene transcription in inflammatory responses. It is also evident that several neurotrophins are induced by extracellular nucleotides and that both proin-flammatory and antiinflammatory genes are induced. Although this may seem puzzling, when we interpret these effects, we should always remember that in some cases, inflammatory molecules are needed to protect cells and restore function [41]. In many organs, including the brain, inflammation is likely to start as a time- and site-specific defence mechanism aimed at eliminating irreversibly damaged or potentially dangerous cells in order to save energy and space for cells that still retain a chance of recovery. When it becomes chronic, inflammation could evolve into a destructive and uncontrolled reaction. Thus, failure to resolve this initial beneficial response could lead to a vicious and anarchic state of chronic activation resulting in brain cell damage and loss [41]. Accumulated data suggest that in a similar way, we should interpret purine-induced gliosis as a defence mechanism that originates to protect neurons from cytotoxic and ischemic insults. Dysregulation of purine-induced gliosis upon chronic inflammatory conditions may be at the basis of the reported deleterious effects induced by purines and pyrimidines under certain experimental paradigms [63]. On this basis, understanding molecular mechanisms and the timing of nucleotide-induced astrogliosis may help exploit the beneficial neuroprotective features of reactive astrocytes while attenuating their harmful properties.
Acknowledgement
Part of the work described here has been supported by the Italian Ministry of Education (Project of National Research Interest PRIN-COFIN 2002 and 2004 and FIRB RBAUO19-ZEN to MPA). Authors are grateful to Prof. Joseph T. Neary, University of Miami, USA, for useful discussion.
Abbreviations
- α,βmeATP
alpha-beta-methylene-ATP
- AA
arachidonic acid
- bFGF
basic fibroblast growth factor
- βγmeATP
beta-gamma-methylene-ATP
- COX-2
cyclooxygenase-2
- ERK1/2
extracellular-regulated kinases 1/2
- GFAP
glial fibrillary acidic protein
- LPS
lipopolysaccharide
- 2meSATP
2-methylthio-ATP
- OPs
oligodendrocyte progenitors
- PLA2
phospholipase A2
- PLC
phospholipase C
- PLD
phospholipase D
- PPADS
pyridoxalphosphate-6-azophenyl-2′4′disulphonic acid
- TNP-ATP
2′3′O-(2,4,6-trinitrophenyl)-ATP
References
- 1.Illes P, Ribeiro JA (2004) Neuronal P2 receptors of the central nervous system. Curr Top Med Chem 4:831′38 [DOI] [PubMed]
- 2.Abbracchio MP, Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64:445′75 [DOI] [PubMed]
- 3.Khakh BS, Burnstock G, Kennedy C et al (2001) International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 53:107′18 [PubMed]
- 4.Abbracchio MP, Boeynaems JM, Barnard EA et al (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24:52′5 [DOI] [PMC free article] [PubMed]
- 5.Weisman GA, Wang M, Kong Q et al (2005) Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol 31:169′83 [DOI] [PubMed]
- 6.Koles L, Furst S, Illes P (2005) P2X and P2Y receptors as possible targets of therapeutic manipulations in CNS illnesses. Drug News Perspect 18:85′01 [DOI] [PubMed]
- 7.Fields RD, Stevens-Graham B (2002) New insights into neuron-glia communication. Science 298:556′62 [DOI] [PMC free article] [PubMed]
- 8.Stevens B, Porta S, Haak LL et al (2002) Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36:855′68 [DOI] [PMC free article] [PubMed]
- 9.Agresti C, Meomartini ME, Amadio S et al (2005) Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50:132′44 [DOI] [PubMed]
- 10.Ishibashi T, Dakin KA, Stevens B et al (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49:823′32 [DOI] [PMC free article] [PubMed]
- 11.Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312′18 [DOI] [PubMed]
- 12.Ferrari D, Chiozzi P, Falzoni S et al (1997) ATP-mediated cytotoxicity in microglial cells. Neuropharmacology 36:1295′301 [DOI] [PubMed]
- 13.Norenberg W, Cordes A, Blohbaum G et al (1997) Coexistence of purino- and pyrimidinoceptors on activated rat microglial cells. Br J Pharmacol 121:1087′098 [DOI] [PMC free article] [PubMed]
- 14.Walz W, Ilschner S, Ohlemeyer C et al (1993) Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci 13:4403′411 [DOI] [PMC free article] [PubMed]
- 15.James G, Butt AM (2002) P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol 447:247′60 [DOI] [PubMed]
- 16.Färber K, Kettenmann H (2005) Physiology of microglial cells. Brain Res Brain Res Rev 48:133′43 [DOI] [PubMed]
- 17.Bianco F, Pravettoni E, Colombo A et al (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174:7268′277 [DOI] [PubMed]
- 18.Boucsein C, Zacharias R, Färber K et al (2003) Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci 17:2267′276 [DOI] [PubMed]
- 19.Bianco F, Fumagalli M, Pravettoni E et al (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Rev 48:144′56 [DOI] [PubMed]
- 20.Tsuda M, Shigemoto-Mogami Y, Koizumi S et al (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:729′30 [DOI] [PubMed]
- 21.Hatten ME, Liem RK, Shelanski ML, Mason CA (1991) Astroglia in CNS injury. Glia 4:233′43 [DOI] [PubMed]
- 22.Pasti L, Volterra A, Pozzan T, Carmignoto G (1997) Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17:7817′830 [DOI] [PMC free article] [PubMed]
- 23.Bezzi P, Volterra A (2001) A neuron-glia signalling network in the active brain. Curr Opin Neurobiol 11:387′94 [DOI] [PubMed]
- 24.Araque A, Carmignoto G, Haydon PG (2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63:795′13 [DOI] [PubMed]
- 25.Akwa Y, Hassett DE, Eloranta ML et al (1998) Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol 161:5016′026 [PubMed]
- 26.Saas P, Boucraut J, Quiquerez AL et al (1999) CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: a key role in brain inflammation? J Immunol 162:2326′333 [PubMed]
- 27.Brambilla R, Abbracchio MP (2001) Modulation of cyclooxygenase-2 and brain reactive astrogliosis by purinergic P2 receptors. Ann N Y Acad Sci 939:54′2 [DOI] [PubMed]
- 28.Centemeri C, Bolego C, Abbracchio MP et al (1997) Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol 121:1700′706 [DOI] [PMC free article] [PubMed]
- 29.Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185′93 [DOI] [PubMed]
- 30.Hassinger TD, Guthrie PB, Atkinson PB et al (1996) An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA 93:13268′3273 [DOI] [PMC free article] [PubMed]
- 31.Guthrie PB, Knappenberger J, Segal M et al (1999) ATP released from astrocytes mediates glial calcium waves. J Neurosci 19:520′28 [DOI] [PMC free article] [PubMed]
- 32.Newman EA, Zahs KR (1997) Calcium waves in retinal glial cells. Science 275:844′47 [DOI] [PMC free article] [PubMed]
- 33.Coco S, Calegari F, Pravettoni E et al (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354′362 [DOI] [PubMed]
- 34.Davalos D, Grutzendler J, Yang G et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752′58 [DOI] [PubMed]
- 35.Fumagalli M, Brambilla R, D’Ambrosi N et al (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43:203′18 [DOI] [PubMed]
- 36.Fumagalli M, Trincavelli L, Lecca D et al (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y(13) receptor. Biochem Pharmacol 68:113′24 [DOI] [PubMed]
- 37.Banfi C, Ferrario S, De Vincenti O et al (2005) P2 receptors in human heart: upregulation of P2X(6) in patients undergoing heart transplantation, interaction with TNFalpha and potential role in myocardial cell death. J Mol Cell Cardiol 39:929′39 [DOI] [PubMed]
- 38.Gallagher CJ, Salter MW (2003) Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci 23:6728′739 [DOI] [PMC free article] [PubMed]
- 39.Ballerini P, Rathbone MP, Di Iorio P et al (1996) Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport 7:2533′537 [DOI] [PubMed]
- 40.Fields RD, Stevens B (2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23:625′33 [DOI] [PubMed]
- 41.Marchetti B, Abbracchio MP (2005) To be or not to be (inflamed)-is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci 26:517′25 [DOI] [PubMed]
- 42.Faulkner JR, Herrmann JE, Woo MJ et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143′155 [DOI] [PMC free article] [PubMed]
- 43.Liberto CM, Albrecht PJ, Herx LM et al (2004) Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89:1092′100 [DOI] [PubMed]
- 44.Neary JT, Rathbone MP, Cattabeni F et al (1996) Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci 19:13′8 [DOI] [PubMed]
- 45.Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G (1994) Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience 59:67′6 [DOI] [PubMed]
- 46.Abbracchio MP, Ceruti S, Langfelder R et al (1995) Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int J Dev Neurosci 13:685′93 [DOI] [PubMed]
- 47.Bolego C, Ceruti S, Brambilla R et al (1997) Characterization of the signalling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br J Pharmacol 121:1692′699 [DOI] [PMC free article] [PubMed]
- 48.Brambilla R, Neary JT, Cattabeni F et al (2002) Induction of COX-2 and reactive gliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signalling. J Neurochem 83:1285′296 [DOI] [PubMed]
- 49.Franke H, Krugel U, Schmidt R et al (2001) P2 receptor-types involved in astrogliosis in vivo. Br J Pharmacol 134:1180′189 [DOI] [PMC free article] [PubMed]
- 50.Brambilla R, Burnstock G, Bonazzi A et al (1999) Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br J Pharmacol 126:563′67 [DOI] [PMC free article] [PubMed]
- 51.Brambilla R, Neary JT, Fumagalli M et al (2003) P2Y receptors in brain astroglial cells: identification of a gliotic P2Y receptor coupled to activation of a calcium-independent Ras/ERK1/2 pathway. Drug Dev Res 59:161′70 [DOI]
- 52.Neary JT, Kang Y, Willoughby KA, Ellis EF (2003) Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23:2348′356 [DOI] [PMC free article] [PubMed]
- 53.Jacques-Silva MC, Rodnight R, Lenz G et al (2004) P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 141:1106′117 [DOI] [PMC free article] [PubMed]
- 54.Neary JT, Kang Y (2005) Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol Neurobiol 31:95′03 [DOI] [PubMed]
- 55.Hu B, Bramlett HM, Sick TJ et al (2001) Activation of ERK/CREB and ATF-2 signalling pathways following traumatic brain injury. J Neurotrauma 18:1161
- 56.John GR, Simpson JE, Woodroofe MN et al (2001) Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: Implications for inflammatory gene expression. J Neurosci 21:4134′142 [DOI] [PMC free article] [PubMed]
- 57.Chorna NE, Santiago-Perez LI, Erb L et al (2004) P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem 91:119′32 [DOI] [PubMed]
- 58.Kim SG, Soltysiak KA, Gao ZG et al (2003) Tumor necrosis factor alpha-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol 65:923′31 [DOI] [PMC free article] [PubMed]
- 59.Ciccarelli R, Di Iorio P, Giuliani P et al (1999) Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia 25:93′8 [DOI] [PubMed]
- 60.Pettifer KM, Kleywegt S, Bau CJ et al (2004) Guanosine protects SH-SY5Y cells against beta-amyloid-induced apoptosis. Neuroreport 15:833′36 [DOI] [PubMed]
- 61.Di Iorio P, Ballerini P, Traversa U et al (2004) The antiapoptotic effect of guanosine is mediated by the activation of the PI 3-kinase/AKT/PKB pathway in cultured rat astrocytes. Glia 46:356′68 [DOI] [PubMed]
- 62.Braun N, Lenz C, Gillardon F et al (1997) Focal cerebral ischemia enhances glial expression of ecto-5-nucleotidase. Brain Res 766:213′26 [DOI] [PubMed]
- 63.Volonte C, Amadio S, Cavaliere F et al (2003) Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord 2:403′12 [DOI] [PubMed]