Abstract
Cardiovascular diseases are the major cause of morbidity and mortality in both men and women in industrially developed countries. These disorders may result from impaired angiogenesis, particularly in response to hypoxia. Despite many limitations, gene therapy is still emerging as a potential alternative for patients who are not candidates for traditional revascularization procedures, like angioplasty or vein grafts. This review focuses on recent approaches in the development of new gene delivery vectors, with great respect to newly discovered AAV serotypes and their modified forms. Moreover, some new cardiovascular gene therapy strategies have been highlighted, such as combination of different angiogenic growth factors or simultaneous application of genes and progenitor cells in order to obtain stable and functional blood vessels in ischemic tissue.
Keywords: Angiogenesis, vascular endothelial growth factor, heme oxygenase, hypoxia inducible factor-1, superoxide dismutase, endothelial progenitor cells
INTRODUCTION
Disorders directly dependent on tissue vascularity, such as coronary artery disease (CAD) or peripheral vascular disease (PVD), still remain a challenging and difficult entity to manage with current surgical and conservative medical approaches. Therefore methods for effective stimulation of new blood vessel formation are urgently needed. Such procedures leading to therapeutic angiogenesis are believed to significantly improve the quality of life and to prevent patients from early death.
There are two major forms of angiogenic factor administration, either as a recombinant human proteins (protein-based therapy) or by gene transfer [Freedman & Isner, 2002]. Despite some beneficial effects of the first strategy on the vasculature [Hendel et al., 2000; Laham et al., 2000], randomized, double-blinded, placebo-controlled clinical trials have generally been disappointing [Henry et al., 2003].
When it turned out to be evident that transfer of therapeutic genes into somatic cells of experimental animals can cure disease, gene therapy became an interesting alternative to the protein-based therapy. Unlike the last one, gene transfer approach can provide the extended presence of the angiogenic factor at foci of limb or myocardial ischemia and long therapeutic effects after a single application with no increase in systemic levels [for a review see: Yla-Herttuala & Alitalo, 2003]. In addition, it gives the possibility for selective local treatment of affected tissues.
Unfortunately, the progress from pioneering studies, which defined the ability to express transgenes in the hearts and vasculature of normal animals, to the development of a phase III clinical study for angiogenesis in patients with stable angina, required more than a decade of research [for reviews see: Pislaru et al., 2002; Yla-Herttuala et al., 2004; Pislaru & Simari, 2005]. After this time, we are still far away from routine clinical application of gene therapy tools. Undeniable, however, current clinical trials have also given many positive hints that these therapies may eventually be effective. Thus, it looks like the therapeutic success of these new approaches is achievable but, some technical problems and basic pharmacologic issues need to be solved and optimized before clinically significant results can be obtained [Yla-Herttuala et al., 2004].
Several conditions apply for successful therapeutic neovascularization. Functional and stable blood vessel formation, that will efficiently supply the ischemic region with oxygenated blood, is among them. Many preclinical studies have been performed in small animal models, such as mice, where transduction efficiency is usually much higher than what can be currently achieved in larger animals or humans [Yla-Herttuala et al., 2004]. Therefore, such conditions, as choice of angiogenic factor, proper route of administration, dose and gene delivery vector, need to be reestablished and optimized.
GENE DELIVERY VECTORS
The choice of an appropriate gene delivery system is the most crucial factor in the development of successful gene therapies. A number of vector systems have evolved over the years and they can be divided into two major groups: non-viral and viral vectors [for a review see: Dulak et al., 2006]. Non-viral vectors comprise plasmids or short-strand nucleic acids (anti-sense, DNA decoys, and small interfering RNA), which are delivered to the cells either in a so-called naked form or with the help of various chemical or physical methods. Plasmids are easy to produce and safe but their main drawback is a very low in vivo transfection efficiency resulting in weak gene expression [Verma & Weitzman, 2005]. Moreover, gene expression from plasmid vectors is transient and usually lasts only for 1-2 weeks, due to the lack of genomic integration and rapid degradation of the plasmids [Yla-Herttuala & Alitalo, 2003].
Plasmid-based angiogenic gene therapy generated a lot of hope. It has been created basing on the promising animal experiments and several uncontrolled clinical trials [for a review see Pislaru & Simari, 2005]. Surprisingly, although the efficiency of plasmid-mediated gene delivery appeared to be very low, further clinical trials have been still designed and carried on. Recently published data from the double-blind, randomized EUROINJECT-ONE trial reported some improvement of cardiac functions in patients receiving intramyocardially VEGF-encoding plasmid by the NOGA-driven mode of delivery [Kastrup et al., 2005]. However, the careful analysis of the data can lead to the supposition, that it is impossible to delineate the effect of VEGF overexpression from placebo. The plasma VEGF level in both control and VEGF-plasmid treated patients before delivery was 70 ± 20 ng/l and 69 ± 14 ng/l, respectively. At one week after injection the VEGF level rose significantly almost two-fold, but the change was very similar both in patients receiving empty plasmid (140 ± 42 ng/l) and plasmid encoding VFGF (140 ± 30 ng/l). Although it has not been demonstrated what was the local expression of growth factor in patients treated with VEGF-encoding plasmid and control vector, the probability that it was different and higher in case of VEGF-plasmid is rather low. The data indicate in fact, that intramyocardial injection of plasmid is such a traumatic event for the heart muscle, that it induces high production of endogenous VEGF.
Similar rise in the level of VEGF, both in control patients and those receiving intramuscular injection of VEGF-encoding plasmid, was observed in another recent, double-blinded randomized study in patients with critical limb ischemia (CLI) [Kusumanto et al., 2006]. Also in this study the primary objective of significant amputation reduction has not been met, although significant and meaningful hemodynamic improvement and improvement in skin ulcers healing has been observed in patients treated with VEGF-plasmid [Kusumanto et al., 2006].
Therefore, all studies in which there were no hard proof of higher expression of growth factor(s) after vector delivery should be analyzed with caution in the light of possibility of potent placebo or treatment-related effects. The very low efficiency of plasmid-mediated gene therapy to human tissues indicates that this mode of direct treatment of heart or skeletal muscle should be rather postponed till the improved approaches will be available.
Viral-based vectors use their natural properties to infect cells, therefore they tend to be a very efficient tool to introduce therapeutic gene. Indeed, the majority of experimental and clinical studies in the field of gene therapy for cardiovascular diseases have shown a great advantage of the use of adenoviral, retroviral and lentiviral vectors.
Retroviral vectors posses the ability to integrate into the genome of the host organism. Two groups of retroviral vectors are being used: “classical” retroviral and lentiviral carriers. The first one is derived from animal oncoretroviruses, such as the murine Moloney leukemia virus. These vehicles were the first vectors used in a gene therapy clinical trial in the early 1990s [Culver et al., 1991] and still constitute the larger proportions of vectors used in clinical trials. It is a consequence of the properties of these vectors, which allow for stable integration into the genome of transduced cells and enable the permanent expression of a transgene. The limitation of oncoretroviral vectors is their inability to transduce nondividing cells and sensitivity to complement cascade, therefore, their transfection efficiency in vivo is very low [Laitinen et al., 1997]. Nevertheless, they can efficiently deliver genes in vitro to proliferating progenitor cells. In addition, this type of modification has enabled the treatment of severe combined immunodeficiency (SCID) in children lacking the gene for common γc chain of cytokine receptor [Cavazzana-Calvo et al., 2000; Cavazzana-Calvo et al., 2004; Cavazzana-Calvo et al., 2005].
Unlike oncoretroviruses, lentiviral vectors can transduce non-dividing cells. Therefore, those carriers found application in delivery to hematopoietic stem cells (HSC) and neural cells [Wiznerowicz & Trono, 2005], but they also may be efficient in transduction of tumor cells [Pellinen et al., 2004] and are particularly considered for gene therapy in relation to HIV infections [Morris et al., 2004].
Some reports pointed at the potential use of lentiviral vectors also in the field of cardiovascular gene therapy, as they were able to efficiently transduce neonatal and adult cardiomyocytes in vitro and in vivo [Sakoda et al., 2003; Zhao et al., 2002]. Nevertheless, their application in humans still raises a lot of controversies, as both categories of retroviral vectors create the risk of insertional mutagenesis owing to random integration into the cellular genome [Schroder et al., 2002]. Although such events have been rarely observed in experimental trials, three cases of leukemia in SCID patients treated with gene therapy highlight the important concerns of the safety of this strategy [Cavazzana-Calvo et al., 2005; Couzin et al., 2005]. Additionally, the risk of lentiviral-induced oncogenesis has recently been underlined [Read et al., 2005] in relation to potential pro-oncogenic effect of woodchuck post-transcriptional regulatory element (WPRE) [Kingsman et al., 2005], which is widely used in many gene therapy vectors in order to enhance transgene expression.
Inflammatory response and transient nature of gene expression are the major limitations in the application of adenoviral (Ad) vectors. They are very efficient in transducing various cell types irrespective of the stage of their cell cycle. In endothelial cells, a transduction efficiency in vivo up to 75% or more has been reported [Gruchala et al., 2004b]. However, the first generation of adenoviral vectors, which have been the most commonly used besides retroviruses in clinical trials [Edelstein et al., 2004], still possesses a significant proportion of adenoviral genes. Therefore, in a short time after transduction, the immune response develops against viral proteins. This response may limit the time and level of expression of therapeutic genes.
PRE-EMPTIVE GENE THERAPY
Sudden occlusion of the blood vessel in the heart or lower limb leads to ischemia/reperfusion (I/R) injury. It refers to paradoxical damage of the tissue caused when blood supply returns to the tissue after a period of ischemia [for a review see: Pasupathy & Homer-Vanniasinkam, 2005]. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage from the oxygen-derived free radicals rather than restoration of normal function of the organ. On the other hand, hypoxic preconditioning defined as brief episodes of ischemia is known to confer cytoprotection against I/R injury [Zhao et al., 2003].
Preconditioning mediated cardioprotection is believed to be achieved through its ability to induce several cardioprotective genes and proteins, including some of the heat shock proteins (Hsp), manganese superoxide dismutase (Mn-SOD), peroxisomal catalase, glutathione peroxidase-1 (GPx-1) and heme oxygenase-1 (HO-1) [Das et al., 1993; Vahlhaus et al., 2005; Yang et al., 2003; Yoshida et al., 2001]. The expression of these stress-inducible and anti-oxidant genes and stimulation of their anti-oxidant enzyme activities may compose the defense system of the heart or limb enabling it to survive against ischemic stress by eliminating the oxidative assault.
The occurrence of I/R is unpredictable. Therefore, pre-emptive administration of a therapeutic gene by a vector that enables long-term expression may provide effective long-term protection against subsequent ischemia and cell death. Adeno-associated viral (AAV) vectors, that were shown to give long-lasting transgene expression can find a broad application in this field. The proof of the concept of a pre-emptive gene therapy has been demonstrated in several studies, in which activation of the expression of protective genes delivered to organs prior to dangerous ischemic event, was either constitutive [Agrawal et al., 2004; Pachori et al., 2006] or regulated by hypoxia [Pachori et al., 2004; Tang et al., 2004; Tang et al., 2005] (Fig. 1).
Fig. (1).
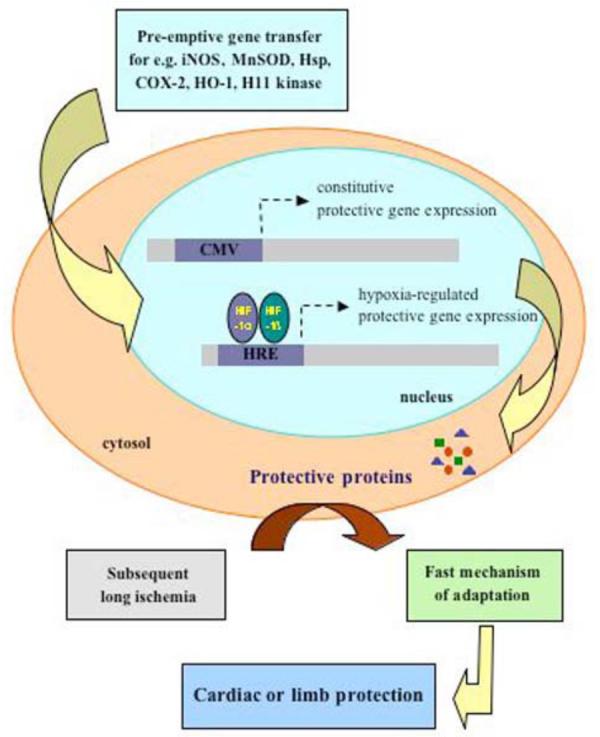
Pre-emptive-gene therapy. The concept of pre-emptive gene therapy was originally based on the protective action of brief episodes of ischemia occurring several hours prior to a myocardial infarction (preconditioning). Proteins up-regulated under such conditions exert anti-oxidant and anti-inflammatory properties, when the subsequent longer ischemia occurs. Pre-emptive administration of a therapeutic gene, like iNOS, MnSOD, Hsp, HO-1, under the control of constitutive cytomegalovirus (CMV) promoter or hypoxia regulated element (HRE), activated by a brief episodes of ischemia prior to myocardial infarction or critical limb ischemia, is believed to confer the fast protective mechanism of adaptation to reperfusion-mediated injury.
Hypoxia-inducible factor-1α (HIF-1α) is a transcriptional activator that functions as a master regulator of cellular and systemic oxygen homeostasis [for reviews see: Maxwell & Ratcliffe, 2002; Zagorska & Dulak, 2004]. In normoxia, HIF-1α is constitutively generated in cells, however, it is immediately degraded. This degradation is dependent on the action of the oxygen-dependent prolyl hydroxylases, which by adding hydroxyl groups to specific proline residues (P402 and P564) present in so-called oxygen-dependent degradation (ODD) domain, creates the signal for von-Hippel-Lindau (VHL) ligase. VHL ubiquitylates HIF-1α, which is targeted for proteasome degradation. Decreased oxygen tension, occurring during tissue ischemia, stabilizes HIF-1α subunit. Stabilized HIF-1α forms heterodimer with HIF-1β, which is also constitutively expressed in cells, but more stable in the presence of oxygen than HIF-1α. These two subunits form a heterodimer that binds to hypoxia responsive element (HRE) present in the regulatory region of many hypoxia-inducible genes, such as vascular endothelial growth factor (VEGF) or erythropoietin. This mechanism allows to switch on adaptation processes, such as induction of angiogenensis, which restores the proper function of damaged ischemic organ.
The concept of protective hypoxia-regulated exogenous gene expression was recently intensively investigated in many experimental studies. Among them, quite interesting strategy, using two plasmid vectors, has been proposed by Tang and coworkers. The sensor plasmid harbored the ODD coding sequence of HIF-1α, inserted between the fragment encoding the yeast GAL4 binding domain (GAL4DBD) and p65 activation domain (p65AD). The effector plasmid encoded the HO-1 gene under the control of 6 copies of GAL4 activation sequence. Plasmids were injected into the mouse heart subjected to myocardial ischemia. The expression of ODD was specific for the cardiomyocytes as it was driven by the myosin light chain (MLC) promoter. Hypoxia stabilized the ODD and resulted in stabilization of the whole activation factor (GAL4DBD-ODD-p65AD), which by binding to the Gal4 binding sequence in the effector plasmid, induced locally the expression of a therapeutic gene in the ischemic heart. HO-1 overexpression attenuated the formation of fibrotic scar, the effect probably being related to the down-regulation of pro-apoptotic Bax, Bak, and caspase 3 levels and upregulation of anti-apoptotic Bcl-2 protein [Tang et al., 2004]. Despite this undoubted protective effects, introduction of the yeast GAL4 protein into humans is rather controversial.
Interestingly, the hypoxia-regulated exogenous gene expression approach has recently been implicated to modify murine mesenchymal stem cells (MSCs). HO-1 harboring vector was able to enhance the tolerance of engrafted MSCs to hypoxia-reoxygenation injury in vitro and improve their viability in ischemic hearts [Tang et al., 2005].
Thus, the concept of hypoxia-induced exogenous gene expression seems to be suitable strategy for the treatment of ischemic diseases. The therapeutic gene is continuously present in the target tissue, but its expression is activated only in response to ischemia and quickly deactivated upon restoration of the perfusion. Nevertheless, one of the potential limitations of this approach may be trapping of the transcription factors by different regulatory sequences delivered to the cells in gene therapy vectors [Dulak et al., 2006].
Agrawal and coworkers [Agrawal et al., 2004] have evaluated the pre-emptive gene therapy using constitutively overexpressed extracellular SOD (EC-SOD, SOD3). AAV-mediated delivery of EC-SOD, driven by cytomegalovirus (CMV) promoter, prevented the injury caused by myocardial infarction induced 6 weeks later. Importantly, analysis performed 16 months after gene transfer demonstrated the persistent increase of functional SOD in the EC-SOD-treated rat ventricles.
In another study, Pachori and coworkers showed that prior delivery of AAV-HO-1 five weeks in advance provides significant protection against recurring multiple I/R injury in rats. Constitutive overexpression of HO-1 was able to prevent myocardial wall thinning, inflammation, fibrosis and aggravation of cardiac function. HO-1–mediated protection after repetitive I/R was associated with decreased apoptosis, due to significant increase in levels of anti-apoptotic proteins, bcl-2 and bcl-xl and reduction in superoxide generation in comparison to LacZ-treated animals [Pachori et al., 2006]. Similar amelioration of the I/R injury in heart, liver and skeletal muscle has been also demonstrated after pre-emptive delivery of AAV containing hypoxia-regulated HO-1 gene (Pachori et al., 2004)
The sort of pre-emptive strategy was also applied by Depre and coworkers. They demonstrated, that transgenic mice with cardiac-specific overexpression of H11 kinase (H11K) were protected against myocardial necrosis and apoptosis as potently as wild type mice were protected by ischemic preconditioning exerted by six episodes of short I/R before a sustained ischemia of 45 minutes. Suggested mechanism responsible for this in vivo cardioprotection by H11K was the activation of survival pathways including Akt and the 5'AMP-activated protein kinase (AMPK), that pre-emptively triggered the anti-apoptotic and metabolic response to ischemia [Depre et al., 2006].
The main advantage of the constitutive promoters, especially in the field of pre-emptive gene therapy, is the quick activation and the high level of expression of the therapeutic protein. On the other hand, once activated gene expression from the constitutive promoter can hardly be “switched off”, what may result in deleterious side-effects related to the excess of produced factor. Moreover, recent data published by Salem and coworkers point at important possible interactions between the major immediate-early enhancer/promoter from human cytomegalovirus (MIEhCMV) and β-adrenergic agonists and antagonists [Salem et al., 2006]. β-adrenergic agonists (β-blockers) are drugs that bind to β-adrenoceptors and thereby block the binding of endogenous catecholamines (norepinephrine and epinephrine) to these receptors, leading to reduction in heart rate, contractility, conduction velocity and relaxation rate. Therefore, β-blockers are drugs of choice for angina, hypertension and heart failure. Intracellular signaling after ligand-mediated activation of β-adrenoceptors is mediated by intracellular adenosine-3',5'-cyclic monophosphate (cAMP). Activation of adenylyl cyclase by G-proteins coupled to the cytosolic domain of the β-adrenoceptor increases cAMP synthesis, which leads to activation of the transcriptional factor cAMP-response element-binding protein (CREB) by protein kinase A (PKA). MIEhCMV contains several copies of the cAMP-response element (CRE) [Montminy, 1997]. CREB binds to CRE and stimulates assembly of the RNA polymerase complex which promotes transcription [Montminy, 1997]. Interestingly, Salem and coworkers have demonstrated that after adenovirus-mediated porcine intracoronary gene transfer, β-blockade mediated by atenolol or propranolol reduced reporter gene expression driven by MIEhCMV by up to 250-fold compared with non-β-blocked animals [Salem et al., 2006]. These data stress the importance of potential interactions between gene therapy and pharmacotherapy.
NEW VECTORS IN GENE THERAPY
As mentioned already, one of the conditions of effective gene therapy is the choice of a proper gene carrier that will efficiently deliver the genetic material to the damaged tissue without causing deleterious side-effects. AAV has emerged as an attractive vector for gene therapy, because of their broad tissue tropism, efficient transduction, and lack of human pathology [for a review see: Grimm & Kleinschmidt, 1999]. AAV vectors are created from small, single-stranded DNA viruses. At least 11 natural serotypes of AAV have been described so far [Cearley & Wolfe, 2006; Gao et al., 2004]. They are termed serotypes 1 – 11 in accordance with their unique serological (immunological) characteristics, and the sequences of isolation and characterization. Some serotypes are highly similar (AAV1 and AAV6), while others show considerable divergence (AAV2 and AAV5) [Blankinship et al., 2004; Wu et al., 2006].
Among all AAV serotypes, AAV2 has been most widely tested in pre-clinical and clinical studies. This provided evidence that AAV2 vectors possess many properties making them very attractive for therapeutic gene delivery to humans and to confer long-term gene expression [for a review see: Grimm & Kay, 2003]. Previously, this long-lasting transgene activity was explained by the ability of the wild-type AAV (wtAAV) to specifically integrate into the clearly defined site on chromosome 19 [Kotin et al., 1990]. Next findings demonstrated, that the integration requires Rep proteins encoded by one of two genes of wtAAV, lacking in the AAV vectors. Thus, rather episomal form of AAV persistence in the transduced tissue is currently suggested [McCarty et al., 2004]. It is believed that not the stable integration, but most probably the ability of AAV vectors to transduce non-dividing cells, such as myocytes or neurons without induction of immune response [Monahan & Samulski, 2000], enables them the long-term gene expression.
Although the majority of AAV vectors remain in episomal forms, AAV vectors can randomly integrate in low proportions into the chromosomal DNA [Kay & Nakai, 2003], probably within DNA regions that are already damaged within treated cells. Despite this random integration, on the basis of data from plenty of normal animals treated with AAV vectors, as well as many clinical trials performed so far, there is no evidence to suggest that AAV gene therapy correlates with increased risk of cancer [Kay & Nakai, 2003].
The application of a new generation of hybrid Ad/AAV vectors was able to restore the ability of AAV vectors to specifically integrate into a specific site (AAVS1) on human chromosome 19q13.3-qter [Recchia et al., 2004]. As mentioned, this specific integration is dependent on the simultaneous expression of rep gene, which so far was omitted from the AAV vectors. A double reporter gene integration cassette, in the study by Recchia and coworkers, flanked by AAV ITRs and tightly regulated, drug-inducible Rep expression cassette, were carried by two different fully deleted helper-dependent adenoviral (HD-Ad) vectors. This Ad/AAV hybrid vector was believed to combine the large capacity and infectivity of adenoviral vectors with the ability of the AAV Rep protein necessary to direct the integration of AAV ITR-flanked sequences at AAVS1 region on chromosome 19. Indeed, this site-specific integration of a double-stranded DNA transgene into the human genome was obtained not only ex vivo in human primary cells, but also upon a single tail vein administration of a nontoxic dose (2 × 108 transducing units) of HD-Ad/AAV vector into transgenic mice carrying one copy of a 3.5-kb fragment of the AAVS1 on the X chromosome. AAVS1-specific integrations were mapped and sequenced in DNA extracted from the livers of animals in which activation of rep expression was induced by drug treatment [Recchia et al., 2004].
Despite the potential of AAV-based applications, a number of limitations need to be overcome. A lot of studies revealed that some cells are refractory to AAV transduction and endothelial cells are among them, with efficacy as low as 2%-5% in case of the AAV2 serotype [Pajusola et al., 2002; Vassalli et al., 2003]. In contrast, efficacy in vascular smooth muscle cells can be as high as 20–40% [Buning et al., 2003; Gruchala et al., 2004a; Richter et al., 2000]. Another very important issue is that gene transfer is hampered by neutralizing anti-AAV2 antibodies, which are highly prevalent in the human population [Erles et al., 1999].
In efforts to overcome these limitations, current investigations focus on the exploitation of other naturally occurring serotypes of AAV. Several preliminary studies have shown different tropism and transduction efficiency of various AAV serotypes on different cell types, when compared to AAV2 [Blankinship et al., 2004; Davidson et al., 2000; Rutledge et al., 1998; Xiao et al., 1999; Zabner et al., 2000], although the primary receptors for most of them are not known. Few years ago, much better efficiency of AAV5 compared with AAV2 in the airways was shown by Zabner and coworkers [Zabner et al., 2000]. Basing on this, sialic acid which is an abundant sugar residue on the apical surface of the airways, was recently suggested to be the primary receptor for AAV serotype 5 [Seiler et al., 2006].
Significant advantage of AAV6 over AAV2 vectors have been shown in in vivo transduction of mouse lung epithelial cells [Halbert et al., 2001], skeletal muscles [Blankinship et al., 2004] and myocardium [Kawamoto et al., 2005]. Other studies demonstrated significant superiority of AAV1 and AAV5 over AAV2 in the efficiency to transduce the heart [Su et al., 2006] or muscle tissues [Chao et al., 2000; Chao et al., 2001; Hildinger et al., 2001; Riviere et al., 2006]. A second administration of a different serotype-based AAV into the immunocompetent mice appears to be fully efficient. Therefore, cross-administration of AAV1, AAV2 and AAV5 is a very promising approach for skeletal muscle gene transfer, as it allows to overcome the risk of low gene transfer efficiency maintained by pre-existing immunity in the host organism due to an initial virus exposure [Riviere et al., 2006].
AAV9 has been recently isolated from human tissues [Gao et al., 2004]. Although the knowledge about the biology of this virus is still limited, it is currently believed to be a robust vector for cardiac gene delivery. AAV9 was shown to transduce myocardium much more efficiently (5- to 10-fold) than AAV8, resulting in over 80% cardiomyocyte transduction. What is even more important, this effect was achieved by systemic delivery through the tail vein injection of 1 × 1011 viral particles (vp) per mouse [Inagaki et al., 2006]. Also, another study, where exactly the same dose (1 × 1011 vp) of AAV2 genome cross-packaged into the AAV9 capsid (AAV2/9) was administered intravenously, showed that adult mice myocardium was readily transduced at the level of approximately 200-fold higher than after AAV2/1 hybrid vector [Pacak et al., 2006].
Another, except myocytes, tempting target for gene therapy of cardiovascular disorders is in vivo expansion of endothelial cells in the vessel wall. Unfortunately, as mentioned already, levels of transduction of endothelial cells by AAV serotype 2 are low. This observation is linked to deficiencies in endothelial cell binding, virion degradation by the proteasome, and/or sequestration of virions in the extracellular matrix rich in heparan sulfate proteoglycan – a primary receptor for AAV2 [Pajusola et al., 2002]. Previous studies suggested that, similarly to muscle gene transfer, it might be possible to improve the transduction efficiency of endothelial cells by using alternate AAV serotypes. Disappointingly however, other AAV serotypes failed to be more effective than AAV2. AAV serotypes 3 through 6 were shown to transduce endothelial cells with poor efficiency [Denby et al., 2005]. Moreover, proteasome degradation was a common limiting factor for endothelial cell transduction also by AAV7 and AAV8 vectors [Denby et al., 2005].
Therefore, current strategies to improve transduction of different cell types, also of endothelial cells, focus mostly on modifications of AAV2 capsid (Fig. 2). According to Choi and coworkers [for a review see: Choi et al., 2005] it can be achieved through: 1) transcapsidation [Hildinger et al., 2001], 2) adsorption of bi-specific antibody to capsid surface [Bartlett et al., 1999], 3) mosaic capsid [Gigout et al., 2005; Rabinowitz et al., 2004], and 4) chimeric capsid [Hauck et al., 2003]. These procedures, especially adsorption of bi-specific antibodies, neutralize wild-type virus tropism and provide a new cell binding capacity. Novel vectors were shown to have a host range different from AAV2, and to escape the anti-AAV2 immune response [for reviews see: Choi et al., 2005; Grimm & Kay, 2003; Niklin & Baker, 2002]. These modified AAV vectors in many cases have enhanced tropism for different tissues and enable organ-specific transgene expression.
Fig. (2).
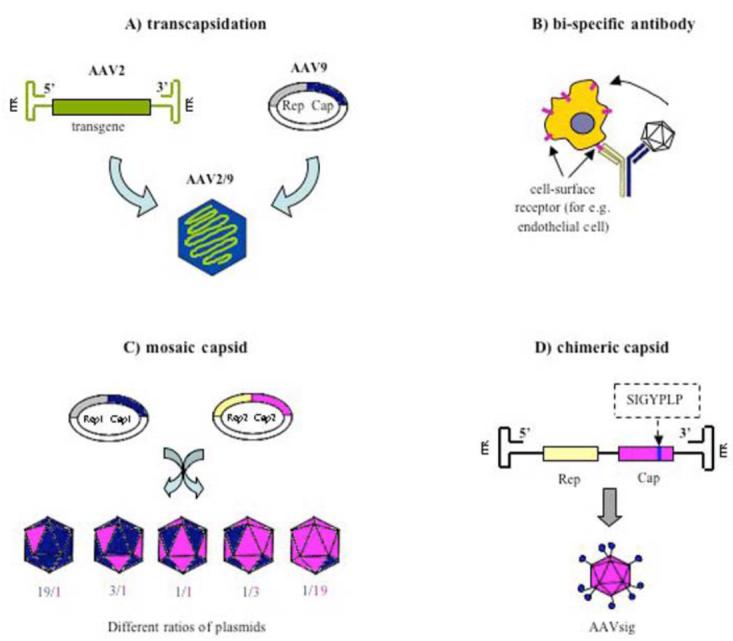
Modifications of AAV2 capsid. All procedures provide new vectors of a new cell-binding capacity, enhanced tissue tropism and ability to escape the anti-AAV2 immune response. A) Transcapsidation (cross-packaging) - packaging AAV genome containing an ITR from one serotype (AAV2) into the capsid of another serotype (AAV9). B) Adsorption of bi-specific antibody made from an AAV-specific antibody, chemically linked to another antibody that binds specifically to a cellular receptor known to be highly expressed on the targeted cell type (for e.g. endothelial cell). C) Mosaic capsid is composed of a mixture of viral capsid proteins from different serotypes (for e.g. AAV1 and AAV2) provided with separate plasmids mixed at a various ratios. During viral assembly, capsid proteins coming from different AAV serotypes are mixed in each virion, at subunit ratios stoichiometrically reflecting the ratios of the complementing plasmids. D) Chimeric capsid is generated by an insertion of a foreign peptide sequence (for e.g. SIGYPLP or QAGTFALRGDNPQG) into the open reading frame of the capsid gene after the position R587 in the Cap sequence.
TARGETED GENE THERAPY
Construction of organ-targeted gene delivery vectors is a promising route to improve the safety and efficacy of gene therapy. The attachment of a receptor-specific ligand to the viral surface increases specificity of the virus to cells of interest. There are various so-called ‘vector targeting’ strategies, which have advanced substantially in the recent past owing to our increasing knowledge of viral biology and pathology as well as the detailing of mechanisms to alter virus–cell binding and/or internalization [Baker et al., 2005]. One of the examples can be human venous endothelial cells-targeting peptides isolated by phage display and genetically incorporated into AAV capsids after amino acid 587. This position has been shown to be optimal for peptide insertion within the capsid to disrupt the interaction of AAV2 to its primary receptor heparan sulphate proteoglycan (HSPG) and to display the inserted peptide on the surface of the virion in order to re-target it for another receptor on the surface of the cell of interest [Nicklin et al., 2001a]. Such SIGYPLP-modified AAV (AAVsig) shows enhanced transduction of human endothelial cells compared with wtAAV capsid, independently of HSPG binding. The increase in gene expression after delivery of 10,000 particles of AAVsig per cell was reported to be about 6-fold higher for primary human umbilical vein endothelial cells (HUVEC) and 28-fold higher for primary human saphenous vein endothelial cells (HSVEC) in comparision to wtAAV [Nicklin et al., 2001a]. The approach of endothelium-binding peptides has been recently verified in vivo. Immunohistochemical analysis of mouse vena cava revealed selective expression of transgene in cells at the luminal surface of peptide modified-AAV but not wtAAV- or PBS-treated animals [White et al., 2004].
In another study, a 14-amino-acid peptide L14 (QAGTFALRGDNPQG), containing an RGD motif, was inserted into the AAV2 cap gene. As RGD is the target for several cellular integrin receptors, this new vector can be used to infect cells displaying αvβ5 integrin, which are otherwise non-permissive to wtAAV2 [Girod A et al., 1999; Shi et al., 2006]. RGD modification of a mosaic AAV1 vector resulted in a 50-100-fold enhancement in endothelial cell gene transfer [Stachler & Bartlett, 2006]. These results suggest that modified AAV vectors hold significant promise for targeted gene delivery to the vasculature.
Another approach of targeted cardiovascular gene therapy is to use promoter/enhancer sequences capable of rendering endothelial- or myocardial-specific transgene expression. Nicklin and coworkers compared three different endothelial cell-specific promoters driving LacZ gene in adenoviral vectors. Among the fms-like tyrosine kinase-1 (Flt-1), intercellular adhesion molecule-2 (ICAM-2) and von Willebrand factor (vWF) promoters, only the first one, Flt-1, gave very high transgene expression and selectivity to in vitro cultured HUVEC cells and intact human vein - transduced ex vivo or after systemic administration [Nicklin et al., 2001b]. Also, endoglin promoter inserted upstream of the human endoglin cDNA expressed high levels of transgene in the endothelium of liver, lung and skin after systemic or local delivery [Velasco et al., 2001]. Moreover, expression of the thrombomodulin gene by AAV under control of the plasminogen activator inhibitor-1 (PAI-1) promoter has been reported to increase transgene expression 600- to 1000-fold compared with endogenous activity in endothelial cells [Mimur et al., 2001].
Selective overexpression of therapeutic genes in the diseased heart creates new strategy for the treatment of cardiovascular disorders. Recombinant adenoviruses containing the β-galactosidase reporter gene under the control of the 250- or 2100-bp rat ventricle-specific cardiac MLC-2v promoter were shown to have high cardiac specificity, when injected into the heart of adult rats in vivo [Griscelli et al., 1998]. Cardiomyocyte-specific overexpression of endothelial nitric oxide synthase (eNOS) inserted downstream of the α-myosin heavy chain (α-MHC) promoter, improved postischemic murine cardiac function by significantly reducing infarct size [Elrod et al., 2006]. Rat cardiomyocytes, infected in vitro with AAV vector encoding VEGF protein under the control of cardiac MLC-2v promoter or the HRE sequence, cultured under anoxic conditions strongly increased VEGF expression. Moreover, ischemic murine hearts injected with this vector had more capillaries and small vessels around the injection site, smaller infarct size, and better cardiac function than the negative controls [Su et al., 2004]. Protective effect of this kind of strategy has been also shown in transgenic animals. Overexpression of HO-1 gene under the control of the α-MHC promoter was shown to be restricted only to the mice heart. This selective overexpression protected hearts subjected to I/R injury mostly by exerting anti-apoptotic effect [Vulapalli et al., 2002].
MULTIGENE APPROACH
The most widely studied growth factors in therapeutic angiogenesis (also in clinical trials) are VEGF-A and basic fibroblast growth factor (bFGF, FGF2) [for a review see: Yla-Herttuala, 2003]. Gene transfer of these two agents, as well as other members of their families has been shown to induce functionally significant angiogenesis in many experimental studies of angiogenic therapy for ischemic heart disease [Giordano et al., 1996; Iwatate et al., 2001; Su et al., 2004] and peripheral arterial disease [Dulak et al., 2002; Rissanen et al., 2003b; Shimpo et al., 2002; Takeshita et al., 1996]. However, lack of expected benefits of this therapy in humans suggests that the stimulation of therapeutic angiogenesis for the treatment of cardiac or limb ischemia with only one growth factor may be insufficient or may potentially lead to tissue edema and death [Isner et al., 2001; Lee et al., 2000]. Moreover, robust expression of VEGF in animal hearts after viral delivery resulted in the formation of unstable and permeable vascular network, that undergoes regression relatively fast [Blau & Banfi, 2001; Dor et al., 2002; Lee et al., 2000]. Therefore, simultaneous induction of angiogenesis and arteriogenesis, which is the process of maturation of preexisting arteriolar collateral connections by their remodeling, would be an optimal clinical outcome. Indeed, the majority of current approaches for therapeutic neovascularization aim at delivering highly effective angiogenic factors to the ischemic regions of limb or heart to simulate vessel sprouting and remodeling of the newly formed capillaries [for a review see: Cao et al., 2005].
Synergistic effect on blood vessel formation of VEGF165 isoform delivered in AAV vector together with angiopoietin-1 (Ang-1), which is required for vessel maturation, was observed by Arsic and coworkers [Arsic et al., 2003]. Adult male Wistar rats showed increased number of CD31- and α-SMA-positive cells and increased perfusion of the tibialis anterior muscle. Moreover, co-expression of VEGF165 with Ang-1, which did not display angiogenic effect per se, remarkably reduced leakage of vessels produced by VEGF165 alone.
Different combinations of other angiogenic growth factors, FGF2, VEGF and platelet-derived growth factor-BB (PDGF-BB), were studied by Cao and coworkers in mouse corneal micropocket assay and rat hindlimb ischemia model [Cao et al., 2003]. In this study, administration of single angiogenic factors, FGF2, PDGF-BB or VEGF was unable to stimulate stable vascular networks. In contrast, combination of FGF2 and PDGF-BB (FGF2/PDGF-BB), but not VEGF and PDGF-BB (VEGF/PDGF-BB) or VEGF and FGF2 (VEGF/FGF2), synergistically induced angiogenesis and functional vessels. The authors suggest, that FGF2, in contrast to VEGF, is able to up-regulate both PDGFR-α and PDGFR-β receptors leading to activation of PDGFR-transduced signaling pathways in newly formed blood vessels and their stabilization. Interestingly, very recently Kano and coworkers have shown, that VEGF together with FGF2 play unique role in synergistic enhancement of endogenous PDGFB – PDGFR-β signaling in mural cells, what promotes mature blood vessel formation in vivo in the Matrigel plug assay [Kano et al., 2005]. Possible explanation of the mural cell recruiting after VEGF/FGF-2 treatment, according to authors suggestions, is the complementary function of both agents. Indeed, PDGF-BB was shown to be induced in endothelial cells by VEGF-A [Arkonac et al., 1998], and FGF-2 stimulated the expression of PDGFR-β in mural cells [Kano et al., 2005]. On the other hand, another report noted selective up-regulation of PDGFR-α, but not of PDGFR-β, in vascular smooth muscle cells by FGF-2 [Schollmann et al., 1992]. Thus, the effect of FGF-2 on the formation of mature blood vessels still needs to be elucidated, as PDGFR-β has only been found in capillaries in contrast to arterioles, which express both the PDGFR-α and the PDGFR-β [Hao et al., 2004].
Kano and coworkers noted significantly weaker effect on the number of mature vessels in the Matrigel plug assay after VEGF/PDGF-BB or FGF2/PDGF-BB in contrast to VEGF/FGF2 application. One of the possible explanations is the effect of overwhelmed endogenous distribution of PDGF-BB by exogenously delivered molecule and in fact impairment in blood vessel stabilization by mural cells [Kano et al., 2005]. Therefore, exogenous PDGF-BB, either sole or in combination with other growth factors, should be used with caution.
Since it is clear that new vascular network formation is a result of precise, “harmonic interplay” of different growth factors, combined multigene approach has gained a lot of attention in the field of cardiovascular gene therapy. However, many additional investigations need to be performed to elucidate the real cross-talk between all of those compounds in order to establish their optimal combinations.
COMBINED CELL- AND GENE THERAPY
Separate Delivery of Vectors and Stem Cells
Cell therapy, similarly to gene therapy, has been proven to be effective to promote neovascularization in various animal models. It has been shown that bone marrow–derived stem/progenitor cells differentiate into circulating endothelial progenitor cells (EPCs), and home to sites of ischemia to contribute to neovascularization, tissue/vessel remodeling, and cardiac regeneration probably by paracrine effects [for a review see: Losordo & Dimmeler, 2004]. Although the exact mechanism regulating differentiation and homing of EPCs still remains unclear, plenty of experimental studies have shown the great advantage of this form of therapy. And as usually, the promising results from animal models promoted the initiation of randomized clinical pilot trials, without (TOPCARE-AMI) [Britten et al., 2003], (BOOST) [Wollert et al., 2004] or with placebo group (REPAIR-AMI) [Schachinger et al., 2006]. These studies have demonstrated the feasibility and safety of administering progenitor cells derived from autologous bone marrow to the myocardium of patients with ischemic heart disease. Cell transfer did not increase the risk of adverse clinical events, in-stent restenosis, or proarrhythmic effects. While clinical efficacy data are still rare, the few controlled trials that have been completed uniformly show a tendency towards better heart function in cell-treated patients [for a review see: Stamm et al., 2006].
Recent reports indicate that cardiologic patients may benefit even more from a combination of stem cell-based and gene therapy. Mobilization of EPCs to the blood occurs after release of cytokines, such as VEGF, stromal derived factor-1 (SDF-1), Ang1 or bFGF, from ischemic tissue. However, this process may be impaired, as the number of EPCs in blood correlates with risk of cardiovascular disorders, such as diabetes [Loomans et al., 2005; Urbich & Dimmeler, 2005], hypercholesterolaemia [Urbich & Dimmeler, 2005] and hypertension [Vasa et al., 2001]. Loss or ageing of EPCs may contribute to incorrect function of blood vessels. Therefore, Shintani and coworkers hypothesized that combining sub-therapeutic doses of cell and gene therapy may allow to overcome the inability of some patients to recruit the EPCs from the bone marrow. Human CD34(+) cells were implanted to ischemic hearts of immunodeficient rats together with local injection of plasmid encoding human vascular endothelial growth factor 2 (phVEGF2, phVEGF-C). Combination therapy was associated with an increased number of circulating EPCs, what contributed to improved fractional shortening, increased capillary density, and reduced infarct size compared with the therapy using only one compound [Shintani et al., 2006].
In another study, application of autologous bone marrow mononuclear cells (BM-MNCs) and injection of plasmid vector encoding Ang-1 was investigated in a rabbit model of operatively induced unilateral hind limb ischemia. Such therapy was able to increase the number of large and small collateral vessels leading to improvement of functional neovascularization determined as the measurement of transcutaneous oxygen pressure and improvement in skin ulcer score. It tends to speculate, that the beneficial effects of such combined therapy may be related to the ability of BM-MNCs to secrete various angiogenic cytokines that harmonize the process of natural neovascularization together with Ang-1 [Kobayashi et al., 2006].
Genetic Modification of Progenitor Cells
EPCs strongly express anti-oxidative enzymes, such as catalase, GPx-1 and MnSOD, which make them resistant to oxidative stress [Dernbach et al., 2004]. Age-related or disease-linked loss of this anti-oxidative potency may result in severe impairment of EPC functions. Meanwhile, experimental deficiency of MnSOD [He et al., 2004] or GPx-1 [Forgione et al., 2002] decreases cell viability, making them more vulnerable to oxidative stress. Moreover, it seems that such changes may result in impairment of growth factor production by EPCs and contribute to the decrease of their vasculogenic potency, what has been observed in GPx-1 deficient mice [Galasso et al., 2006].
The genetic modification of EPCs may overcome this loss of function and improve the efficiency of cell-based therapeutic angiogenesis. There are efforts in order to create the progenitor cells not only of a better redox phenotype, but also of more efficient angiogenic potential. Accordingly, ex vivo transfection of endothelial progenitor cells with VEGF enhanced EPC proliferation, adhesion, and incorporation into endothelial cell monolayers in vitro [Iwaguro et al., 2002]. Moreover, these VEGF-modified EPCs augmented naturally impaired neovascularization in an animal model of experimentally induced limb ischemia.
Recently, efforts have been also made to dissect the regulatory pathways that control the phenotypes of stem cell populations. Some of them focused on the role of glycogen synthase kinase-3β (GSK3β), which is a serine/threonine kinase under the control of Wnt and phosphoinositide 3-kinase/Akt signaling pathways [Choi et al., 2004]. This protein has been shown to be involved in the negative regulation of angiogenesis through its ability to modulate vascular endothelial cell migration and survival [Kim et al., 2002]. In the study by Choi and coworkers catalytically inactive GSK3β (GSK3β-KM) was used to modify EPC from human peripheral blood. Such genetically modified EPC displayed enhanced proliferation, survival, and differentiation in vitro and enhanced vasculogenic potential in vivo [Choi et al., 2004]. These effects were probably mediated by increased VEGF and IL-8 expression.
Other Approaches
VEGF and FGF family members were the most widely studied growth factors in experimental and clinical trials of therapeutic angiogenesis. Nevertheless, there are plenty of other angiogenic agents, the role of which in the process of new blood vessels formation has been already well documented in experimental studies (Table 1). As the choice of the proper angiogenic growth factor is one of the conditions of the successful gene transfer, their investigation in large gene therapy trials would be also reasonable.
Table 1.
Examples of the Recent Experimental Studies for Cardiovascular Gene Therapy
| Therapeutic gene |
Carrier/ Mode of regulation |
Delivery | Application | Species | Therapeutic effect | References | |
|---|---|---|---|---|---|---|---|
|
Pre-emptive/ regulatable gene therapy |
HO-1 | Plasmid/ cardiac specific and hypoxia-regulated AAV/ constitutive (CMV) or hypoxia- regulated |
Intramyocardial injection Intramyocardial injection |
Myocardial infarction (I/R injury) Myocardial infarction (I/R injury) |
mouse rat |
attenuation of the formation of fibrotic scar amelioration of cardiac function – prevention of myocardial wall thining, inflammation and fibrosis |
Tang et al., 2004 Pachori et al., 2004; 2006 |
| VEGF | AAV/ Cardiac-specific and hypoxia-regulated |
Intramyocardial injection |
Ischemic heart | mouse | higher VEGF expression in ischemic heart, increased neovasculogenesis, less myocardial fibrosis, better cardiac fuction |
Su et al., 2004 | |
| SOD-3 (EC- SOD) |
AAV/ constitutive (CMV) promoter |
Intramyocardial injection |
Myocardial infarction (I/R injury) |
rat | decrease in infarct size 24h after I/R injury, improved ventricular function, enhanced survival |
Agrawal et al., 2004 | |
|
Novel proangiogenic genes |
VEGF-D | Ad/ constitutive (CMV) promoter |
Intramuscular injection |
Ischemic hindlimb |
rabbit | increased mean capillary area and vascular permeability, promotion of VSMC proliferation (arteriogenesis) |
Rissanen et al., 2003 |
| Kallikrein | Ad/ Constitutive (CMV) promoter |
Intramuscular injection |
Ischemic and non-ischemic hindlimb |
mouse | increased capillary density, enhanced perfusion |
Emanueli et al., 2000; 2001 |
|
| Multigene approach: VEGF and Ang-1 Human CD34+ progenitor cells and VEGF-C |
AAV/ constitutive (CMV) promoter plasmid/constitutive (CMV) promoter |
Intramuscular injection Intramyocardial injection |
Non-ischemic tibialis anterior muscle Ischemic heart |
rat immunodeficient rat |
Increased number of mature blood vessels Increased capillary density, reduced infarct size |
Arsic et al., 2003 Shintani et al., 2006 |
|
|
Activation of endogenous genes |
HIF-1α | Plasmid (HIF-1α/VP16 hybrid gene) AAV (HIF-1α without transactivation domain VP16) |
Intramyocardial injection Intramuscular injection |
Ischemic heart (acute myocardial infarction) Non-ischemic hindlimb |
rat mouse |
Enhanced angiogenesis, reduced infarct size increased capillary sprouting and proliferation, no increase in vascular leakage in the transduced muscle, enhanced perfusion |
Shyu et al., 2002 Pajusola et al., 2005 |
| PR39 | Ad/ constitutive (CMV) promoter |
Intramyocardial catheterization |
Chronically ischemic hart |
pig | increased vessel size and number, improved myocardial perfusion and function |
Post et al., 2006 | |
|
Modification of progenitor cells: PB-EPC: autologous human MSCs: |
eNOS or HO-1 |
Retroviral vector/ 5'LTR promoter |
Catheter mediated instillation of EPCs |
Balloon injury of the right common carotid artery |
rabbit |
enhanced reendotheliazation independently of EPC modification, reduced neointima size after delivery of eNOS-modified EPC |
Kong et al., 2004 |
| GSK3β (inactive form) |
Ad/constitutive | Direct cell injection into systemic arterial circulation |
Ischemic hindlimb |
immunodeficient mouse |
Improved blood flow, limb salvage and tissue capillary density |
Choi et al., 2004 | |
| HO-1 | Plasmid/ hypoxia-regulated promoter |
Intramyocardial injection |
Ischemic heart (acute myocardial infarction) |
mouse | Improved viability of mesenchymal stem cells |
Tang et al., 2005 |
Hepatocyte growth factor (HGF), although originally identified as a potent mitogen for hepatocytes [Strain et al., 1982], induces angiogenesis in various animal models through exerting mitogenic action also on endothelial cells [for a review see: Morishita et al., 2004]. Currently ongoing double-blinded, placebo-controlled HGF-STAT clinical trial tests the effectiveness of plasmid DNA containing HGF in the treatment of critical limb ischemia [Powell et al., 2004].
VEGF-D is a member of VEGF growth factors family and it is synthesized and secreted as a large precursor form that is proteolytically processed into mature form (VEGF-D N C) comprising the central VEGF homology domain [Stacker et al., 1999]. Unprocessed form of VEGF-D preferentially signals through VEGFR-3 leading to the induction of lymphangiogenesis, but the mature form efficiently triggers VEGFR-2 signaling and stimulates blood vessel formation. Adenoviral administration of VEGF-DΔNΔC to the ischemic rabbit hindlimb resulted in a strong increase in the mean capillary area and vascular permeability, pointing at angiogenic action of this agent very similar to VEGF-A [Rissanen et al., 2003a].
The inability to generate collateral vessels in many patients with chronic vascular insufficiency may be associated with a failure to appropriately increase angiogenic growth factor production with hypoxia or ischemia [Schultz et al., 1999]. Therefore, stimulation of endogenous gene expression by the application of transcription factors, such as HIF-1α, may overcome this impairment, leading to efficient angiogenesis. Constitutive overexpression of a hybrid protein consisting of DNA-binding and dimerization domains from the HIF-1α subunit and the transactivation domain from herpes simplex virus VP16 protein (HIF-1α/VP16) was able to induce angiogenesis in various animal models of ischemic skeletal muscles and myocardium [Vincent et al., 2000; Shyu et al., 2002; Pajusola et al., 2005]. Recently, Luo and coworkers have shown that Ad2/HIF-1α/VP16 was able to activate HRE sequence in the promoter of brain natriuretic peptide (BNP) [Luo et al., 2006]. As BNP is known to be cardioprotective, this study provides support for the therapeutic use of the chimeric HIF-1α/VP16 protein in coronary heart disease. Nevertheless, still there is a need for additional, detailed investigation, as HIF-1α is a key regulatory molecule that acts upon a large number of downstream gene networks. As suggested by Wilhide and Jones, particularly useful would be comprehensive gene expression profiling coupled with functional analysis of HIF-1α/VP16-regulated genes. The results of such studies will elucidate the mechanism of beneficial effects and address concerns regarding potential adverse effects of activating specific HIF-1α/VP16-dependent gene programs [Wilhide & Jones, 2006].
Another promising approach is the use of a macrophage-derived peptide, PR39, that was shown to inhibit the ubiquitin-proteasome-dependent degradation of HIF-1α protein, leading to accelerated formation of vascular structures in vitro [Li et al., 2000]. Moreover, PR39 overexpression in murine hearts under the control of the MHC promoter, as well as subcutaneous implantation of this agent mixed with Matrigel resulted in robust angiogenesis. In study by Post and coworkers adenoviral delivery of this arginine-rich peptide (Ad-PR39) into the chronically ischemic pig myocardium, resulted in local induction of VEGF and FGF signaling pathways. Up-regulation of VEGF, FGFR-1 and syndecan-4 was associated with the increased vessel size and number, what further improved perfusion of the myocardium and increased the target wall motion pointing at the functional improvement of myocardial contractility [Post et al., 2006]. Thus, at this point, it is hard to clearly define, whether observed effect was mediated by induction of HIF-1α or other up-regulated agents, like FGFR-1. Final proof of the real efficacy of PR39 protein, as well as stabilized HIF1α , in humans awaits carefully designed randomized, double-blinded, placebo-controlled clinical trials.
Several years ago, the concept has been introduced that in the setting of ischemia, vascular and neural factors may cooperate to promote tissue repair. Indeed, while the significant role of VEGF in angiogenesis is unquestioned, recent studies show some direct effects of VEGF and its both receptors on the nervous system in terms of neuronal growth, survival, axonal outgrowth, and neuroprotection.
Using two different animal models of diabetes Schratzberger and coworkers have shown, that angiogenic growth factors may constitute a novel treatment strategy for diabetic peripheral neuropathy. VEGF overexpression restored proper vascularization of diabetes-affected nerves and resulted in restoration of large and small fiber peripheral nerve function [Schratzberger et al., 2001]. Additionally, VEGF gene therapy was reported to have a direct neuroprotective effects on motoneurons in vivo and prolonged the progression of amyotrophic lateral sclerosis (ALS) and survival of SOD1(G93A) mice, an animal model of ALS [Azzouz et al., 2004].
Moreover, VEGF may act as a neutrotrophic factor and can produce neurogenic effects on neuronal progenitors. In this regard, Cao and coworkers have recently reported that neuronal expression of VEGF in the rat hippocampus enhanced neurogenesis and that the phenomenon was associated with improved cognition independent of endothelial cell proliferation [Cao et al., 2004].
On the other hand, nerve growth factor (NGF) which regulates neuron survival and differentiation has been implicated in the promotion of new blood vessels formation. Continuous treatment with NGF protein for 14 days enhanced the spontaneous neovascularization of ischemic adductors, encompassing increased capillary sprouting and arteriole growth [Emanueli et al., 2002]. Amplification of reparative neovascularization accelerated the rate of local perfusion in the mice adductor muscle to the levels recorded before ischemia. This local increase in capillary density has been suggested to be associated with the activation of the VEGF-Akt-NO–dependent pathway, as in normoperfused muscles NGF-induced capillarization was blocked by VEGF–neutralizing antibodies, dominant-negative Akt, or NO synthase inhibition [Emanueli et al., 2002]. It remains to be established whether similar efficiency can be obtained with NGF gene transfer.
Thus, the pleiotropic effects of both VEGF and NGF in the cardiovascular and nervous system may initiate some new ideas for the treatment of neurodegenerative diseases, such as Alzheimer's and Parkinson's, as well as of numerous angiogenesis-dependent diseases, such as cancer, arthritis and diabetes.
Another agent of pleiotropic function that has been shown to possess potent angiogenic effects is kallikrein, a member of the serine proteinase superfamily. Kinin peptides formed by the enzymatic cleavage of kininogen by kallikrein bind G-protein coupled subtype B1 and B2 receptors. Activation of kinin receptors, through stimulation of nitric oxide–cGMP and prostacyclin-cAMP pathways, modulates a broad spectrum of biological functions, such as regulation of local and systemic hemodynamics, vascular permeability, electrolyte and glucose transport, preservation of muscular energy content [Emanueli et al., 2000]. Moreover, this protein has been found to exert proliferative effects on endothelial cells via an IP3K-Akt-NO mediated mechanism independently of VEGF [Emanueli et al., 2004]. Intramuscular delivery of adenovirus encoding human kallikrein gene under the control of the CMV promoter (AdCMVHK) enhanced the capillary density in normoperfused mice skeletal muscle [Emanueli et al., 2000], as well as accelerated the spontaneous angiogenesis caused by ischemia [Emanueli et al., 2001].
CONCLUSIONS
Despite undeniable success of proangiogenic gene therapy in many experimental studies, clinical benefits still do not fulfill the expectations. Plenty of different agents can be responsible for that, just to mention an inefficient therapeutic agent, a wrong dose, a less-than-optimal route of administration, an inefficient delivery system, or insufficient duration of the treatment [for a review see: Yla-Herttuala et al., 2004]. Optimization of these basic issues is therefore of great importance for clinically successful vascular growth factor therapy.
A big step forward would be, unquestionable, the finding of a proper carrier, efficiently delivering therapeutic genes into the target tissue of the host organism. AAV vectors still hold this great promise, especially in the light of recent findings showing the great superiority of the newly discovered AAV serotypes over AAV2 that have been most widely used so far.
The concept of pre-emptive gene therapy exerted by proteins with anti-oxidant and anti-inflammatory properties is a novel approach offering exciting possibilities. It is therefore suggested that in future the pre-emptive myocardial gene therapy may find its utility in selected high-risk patients undergoing an interventional or a surgical revascularization procedures [Agrawal et al., 2004]. Current efforts in order to create the progenitor cells of a better redox and angiogenic phenotype, makes the therapy even more attractive, as it may confer cardioprotection and initiate the repair process at the same time.
ACKNOWLEDGMENTS
Research on gene therapy are supported by grants PBZ-KBN 096/P04/2004, 2P04 016 26 and 1998/P01/2006/31 from the Ministry of Science and Informatics Technology. A. Jazwa is the scholar of the young scientists scholarship from the Stanislaw Estreicher Foundation of the Jagiellonian University. A. Jozkowicz is the Wellcome Trust International Research Senior Fellow in Biomedical Science. We thank Andrzej Rutkowski for help with figures.
LIST OF ABBREVIATIONS
- α-MHC
α-Myosin heavy chain
- AAV
Adeno-associated viral vectors
- AAVsig
SIGYPLP-modified AAV
- Ad
Adenoviral vectors
- ALS
Amyotrophic lateral sclerosis
- AMPK
5'AMP-activated protein kinase
- Ang-1
Angiopoietin-1
- bFGF, FGF2
Basic fibroblast growth factor
- BM-MNCs
Bone marrow mononuclear cells
- BNP
Brain natriuretic peptide
- CAD
Coronary artery disease
- cAMP
Adenosine-3',5'-cyclic monophosphate
- cGMP
Guanosine-3',5'-cyclic monophosphate
- CLI
Critical limb ischemia
- CMV
Cytomegalovirus
- COX-2
Cyclooxygenase-2
- CRE
cAMP-response element
- CREB
cAMP-response element-binding protein
- EC-SOD, SOD3
Extracellular superoxide dismutase
- eNOS
Endothelial nitric oxide synthase
- EPCs
Endothelial progenitor cells
- FGFR-1
Fibroblast growth factor receptor-1
- Flt-1
Fms-like tyrosine kinase-1
- GPx-1
Peroxisomal catalase, glutathione peroxidase-1
- GSK3β
Glycogen synthase kinase-3β
- H11K
H11 kinase
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia-inducible factor-1α
- HO-1
Heme oxygenase-1
- HRE
Hypoxia responsive element
- HSCs
Hematopoietic stem cells
- Hsp
Heat shock proteins
- HSPG
Heparan sulphate proteoglycan
- HSVEC
Human saphenous vein endothelial cells
- HUVEC
Human umbilical vein endothelial cells
- iNOS
Inducible nitric oxide synthase
- I/R
Ischemia/reperfusion
- ICAM-2
Intercellular adhesion molecule-2
- MHC
Myosin heavy chain
- MIEhCMV
Major immediate-early enhancer/promoter from human cytomegalovirus
- MLC
Myosin light chain
- Mn-SOD
Manganese superoxide dismutase
- MSC
Mesenchymal stem cells
- NGF
Neural growth factor
- ODD
Oxygen-dependent degradation domain
- PB-EPCs
Peripheral blood endothelial progenitor cells
- PAI-1
Plasminogen activator inhibitor-1
- PDGF-BB
Platelet-derived growth factor-BB
- PDGFR-α
Platelet-derived growth factor receptor α
- PDGFR-β
Platelet-derived growth factor receptor β
- phVEGF2
Plasmid encoding human vascular endothelial growth factor 2 (VEGF-C)
- PKA
Protein kinase A
- PVD
Peripheral vascular disease
- SCID
Severe combined immunodeficiency
- SDF-1
Stromal derived factor-1
- VEGF-A, VEGF
Vascular endothelial growth factor-A
- VEGF-DΔNΔC
Mature form of vascular endothelial growth factor-D
- VEGFR-2
Vascular endothelial growth factor receptor-2
- VEGFR-3
Vascular endothelial growth factor receptor-3
- VHL
Von-Hippel-Lindau
- VSMC
Vascular smooth muscle cells
- vWF
Von Willebrand factor
- WPRE
Woodchuck post-transcriptional regulatory element
- wtAAV
Wild-type adeno-associated viral vectors
REFERENCES
- Agrawal RS, Muangman S, Layne MD, Melo L, Perrella MA, Lee RT, Hang L, Lopez-Ilasaca M, Dzau VJ. Pre-emptive gene therapy using recombinant adenoassociated virus delivery of extracellular superoxide dismutase protects heart against ischemic reper-fusion injury, improves ventricular function and prolongs survival. Gene Ther. 2004;11:962–969. doi: 10.1038/sj.gt.3302250. [DOI] [PubMed] [Google Scholar]
- Arkonac BM, Foster LC, Sibinga NE, Patterson C, Lai K, Tsai JC, Lee ME, Perrella MA, Haber E. Vascular endothelial growth factor induces heparin-binding epidermal growth factor-like growth factor in vascular endothelial cells. J Biol Chem. 1998;273:4400–4405. doi: 10.1074/jbc.273.8.4400. [DOI] [PubMed] [Google Scholar]
- Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, Salvi A, Sinagra G, Giacca M. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003;7:450–459. doi: 10.1016/s1525-0016(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkerbaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Baker AH, Kritz A, Work LM, Nicklin SA. Cell-selective viral gene delivery vectors for the vasculature. Exp Physiol. 2005;90:27–31. doi: 10.1113/expphysiol.2004.028126. [DOI] [PubMed] [Google Scholar]
- Bartlett JS, Kleinschmidt J, Boucher RC, Samulski RJ. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab'gamma)2 antibody. Nat Biotechnol. 1999;17:181–186. doi: 10.1038/6185. [DOI] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, Miller DA, Chamberlain JS. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Blau HM, Banfi A. The well-tempered vessel. Nat Med. 2001;7:532–534. doi: 10.1038/87850. [DOI] [PubMed] [Google Scholar]
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCAREAMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- Buning H, Nicklin SA, Perabo L, Hallek M, Baker AH. AAV-based gene transfer. Curr Opin Mol Ther. 2003;5:367–375. [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cao Y, Hong A, Schulten H, Post MJ. Update on therapeutic neovascularization. Cardiovasc Res. 2005;65:639–648. doi: 10.1016/j.cardiores.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Fischer A. Efficacy of gene therapy for SCID is being confirmed. Lancet. 2004;364:2155–2156. doi: 10.1016/S0140-6736(04)17603-4. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Lagresle C, Hacein-Bey-Abina S, Fischer A. Gene therapy for severe combined immunodeficiency. Annu Rev Med. 2005;56:585–602. doi: 10.1146/annurev.med.56.090203.104142. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Chao H, Monahan PE, Liu Y, Samulski RJ, Walsh CE. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol Ther. 2001;4:217–222. doi: 10.1006/mthe.2001.0449. [DOI] [PubMed] [Google Scholar]
- Choi JH, Hur J, Yoon CH, Kim JH, Lee CS, Youn SW, Oh IY, Skurk C, Murohara T, Park YB, Walsh K, Kim HS. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biol Chem. 2004;279:49430–49438. doi: 10.1074/jbc.M402088200. [DOI] [PubMed] [Google Scholar]
- Choi VW, McCarty DM, Samulski RJ. AAV hybrid sero-types: improved vectors for gene delivery. Curr Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin J, Kaiser J. Gene therapy. As Gelsinger case ends, gene therapy suffers another blow. Science. 2005;307:1028. doi: 10.1126/science.307.5712.1028b. [DOI] [PubMed] [Google Scholar]
- Culver KW, Osborne WR, Miller AD, Fleisher TA, Berger M, Anderson WF, Blaese RM. Correction of ADA deficiency in human T lymphocytes using retroviral-mediated gene transfer. Transplant Proc. 1991;23:170–171. [PubMed] [Google Scholar]
- Das DK, Engelman RM, Kimura Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischemia. Cardiovasc Res. 1993;27:578–84. doi: 10.1093/cvr/27.4.578. [DOI] [PubMed] [Google Scholar]
- Davidson B, Stein C, Heth J, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denby L, Nicklin SA, Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Anti-oxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, Goelman G, Keshet E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak J, Partyka L, Jozkowicz A, Heba G, Prager M, Neumayer C, Sobhian B, Thurner M, Nanobashvili J, Fuegl A, Ratajska A, Polterauer P, Pachinger O, Weidinger F, Dembinska-Kiec A, Redl H, Huk I. Gene transfer of naked vegf plasmid induces the formation of microvessels but not mature collaterals in ischemic limb muscles. Eur Surg. 2002;34:105–110. [Google Scholar]
- Dulak J, Zagorska A, Wegiel B, Loboda A, Jozkowicz A. New strategies for cardiovascular gene therapy: regulatable pre-emptive expression of pro-angiogenic and antioxidant genes. Cell Biochem Biophys. 2006;44:31–42. doi: 10.1385/CBB:44:1:031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004 – an overview. J Gene Med. 2004;6:597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, Janssens S, Feelisch M, Lefer DJ. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocar-dial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–1523. doi: 10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Gozzi MG, Smith R, Gaspa L, Bianchini G, Stillo F, Capogrossi MC, Madeddu P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre JS, Clergue M, Figueroa CD, Gadau S, Condorelli G, Madeddu P. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Zacheo A, Minasi A, Chao J, Chao L, Salis MB, Stacca T, Straino S, Capogrossi MC, Madeddu P. Adenovirus-mediated human tissue kallikrein gene delivery induces angiogenesis in normoperfused skeletal muscle. Arterioscler Thromb Vasc Biol. 2000;20:2379–2385. doi: 10.1161/01.atv.20.11.2379. [DOI] [PubMed] [Google Scholar]
- Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J Med Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Forgione MA, Weiss N, Heydrick S, Cap A, Klings ES, Bierl C, Eberhardt RT, Farber HW, Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med. 2002;(1):54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigout Lm, Rebollo Pm, Clement Nm, Warrington KH, Jr, Muzyczka N, Linden RM, Weber T. Altering AAV tropism with mosaic viral capsids. Mol Ther. 2005;11:856–865. doi: 10.1016/j.ymthe.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, Mathieu-Costello O, Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, Deleage G, Hallek M. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nature Med. 1999;5:1052–1056. doi: 10.1038/12491. http://ep.physoc.org/cgi/external_ref?access_num=10.1038/12491&link_type=DOI. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kleinschmidt JA. Progress in adeno-associated virus type 2 vector production: promises and prospects for clinical use. Hum Gene Ther. 1999;10:2445–2450. doi: 10.1089/10430349950016799. [DOI] [PubMed] [Google Scholar]
- Griscelli F, Gilardi-Hebenstreit P, Hanania N, Franz WM, Opolon P, Perricaudet M, Ragot T. Heart-specific targeting of beta-galactosidase by the ventricle-specific cardiac myosin light chain 2 promoter using adenovirus vectors. Hum Gene Ther. 1998;9:1919–1928. doi: 10.1089/hum.1998.9.13-1919. [DOI] [PubMed] [Google Scholar]
- Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, Kholova I, Markkanen JE, Rutanen J, Heikura T, Alitalo K, Bueler H, Yla-Herttuala S. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004a;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- Gruchala M, Roy H, Bhardwaj S, Yla-Herttuala S. Gene therapy for cardiovascular diseases. Curr Pharm Des. 2004b;10:407–423. doi: 10.2174/1381612043453379. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Mansson-Broberg A, Gustafsson T, Grinnemo KH, Blomberg P, Siddiqui AJ, Wardell E, Sylven C. Angiogenic effects of dual gene transfer of bFGF and PDGF-BB after myocardial infarction. Biochem Biophys Res Commun. 2004;315:1058–1063. doi: 10.1016/j.bbrc.2004.01.165. [DOI] [PubMed] [Google Scholar]
- Hauck B, Chen L, Xiao W. Generation and characterization of chimeric recombinant AAV vectors. Mol Ther. 2003;7:419–425. doi: 10.1016/s1525-0016(03)00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Effect of intra-coronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. VIVA Investigators. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner JM, Vale PR, Symes JF, Losordo DW. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res. 2001;89:389–400. doi: 10.1161/hh1701.096259. [DOI] [PubMed] [Google Scholar]
- Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- Iwatate M, Miura T, Ikeda Y, Kawamura S, Dairaku Y, Okamura T, Kimura M, Yamaguchi K, Ueno H, Matsuzaki M. Effects of in vivo gene transfer of fibroblast growth factor-2 on cardiac function and collateral vessel formation in the microembolized rabbit heart. Jpn Circ J. 2001;65:226–231. doi: 10.1253/jcj.65.226. [DOI] [PubMed] [Google Scholar]
- Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazono K, Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, Botker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyongyosi M, Sylven C, Euroinject One Group Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Shi Q, Nitta Y, Miyazaki J, Allen MD. Widespread and early myocardial gene expression by adeno-associated virus vector type 6 with a beta-actin hybrid promoter. Mol Ther. 2005;11:980–985. doi: 10.1016/j.ymthe.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Kay MA, Nakai H. Looking into the safety of AAV vectors. Nature. 2003;424:251. doi: 10.1038/424251b. [DOI] [PubMed] [Google Scholar]
- Kim HS, Skurk C, Thomas SR, Bialik A, Suhara T, Kureishi Y, Birnbaum M, Keaney JF, Jr, Walsh K. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem. 2002;277:41888–41896. doi: 10.1074/jbc.M206657200. [DOI] [PubMed] [Google Scholar]
- Kingsman SM, Mitrophanous K, Olsen JC. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kondo T, Inoue N, Aoki M, Mizuno M, Komori K, Yoshida J, Murohara T. Combination of in vivo angiopoietin-1 gene transfer and autologous bone marrow cell implantation for functional therapeutic angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1465–1472. doi: 10.1161/01.ATV.0000223865.64812.26. [DOI] [PubMed] [Google Scholar]
- Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, McLaughlin S, Muzyczka N, Rocchi M, Berns KI. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumanto YH, Van Weel V, Mulder NH, Smit AJ, Van den Dungen JJAM, Hooymans JMM, Sluiter WJ, Tio RA, Quax PHA, Gans ROB, Dullaart RPF, Hospers GAP. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Human Gene Ther. 2006;17::683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, Pettigrew RI, Whitehouse MJ, Yoshizawa C, Simons M. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36:2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Pakkanen T, Donetti E, Baetta R, Luoma J, Lehtolainen P, Viita H, Agrawal R, Miyanohara A, Friedmann T, Risau W, Martin JF, Soma M, Yla-Herttuala S. Gene transfer into the carotid artery using an adventitial collar: comparison of the effectiveness of the plasmid-liposome complexes, retroviruses, pseudotyped retroviruses, and adenoviruses. Hum Gene Ther. 1997;8:1645–1650. doi: 10.1089/hum.1997.8.14-1645. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. PR39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49–55. doi: 10.1038/71527. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, De Koning EJ, Staal FJ, Rabelink TJ, Zonneveld AJ. Endothelial progenitor cell dysfunction in type 1 diabetes: another consequence of oxidative stress? Antioxid Redox Signal. 2005;7:1468–1475. doi: 10.1089/ars.2005.7.1468. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- Luo Y, Jiang C, Belanger AJ, Akita GY, Wadsworth SC, Gregory RJ, Vincent KA. A constitutively active hypoxiainducible factor-1alpha/VP16 hybrid factor activates expression of the human B-type natriuretic peptide gene. Mol Pharmacol. 2006;69:1953–1962. doi: 10.1124/mol.105.017905. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Mimur J, Muramatsu S, Hakamada Y, Mori K, Kikuchi J, Urabe M, Madoiwa S, Ozawa K, Sakata Y. Recombinant adeno-associated virus vector-transduced vascular endothelial cells express the thrombomodulin transgene under the regulation of enhanced plasminogen activator inhibitor-1 promoter. Gene Ther. 2001;8:1690–1697. doi: 10.1038/sj.gt.3301579. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7:24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Morishita R, Aoki M, Hashiya N, Yamasaki K, Kurinami H, Shimizu S, Makino H, Takesya Y, Azuma J, Ogihara T. Therapeutic angiogenesis using hepatocyte growth factor (HGF) Curr Gene Ther. 2004;4:199–206. doi: 10.2174/1566523043346453. [DOI] [PubMed] [Google Scholar]
- Morris KV, Rossi JJ. Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection. Curr HIV Res. 2004;2:185–191. doi: 10.2174/1570162043484906. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Baker AH. Tropism-modified adenoviral and adeno-associated viral vectors for gene therapy. Curr Gene Ther. 2002;2:273–93. doi: 10.2174/1566523023347797. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Buening H, Dishart KL, de Alwis M, Girod A, Hacker U, Thrasher AJ, Ali RR, Hallek M, Baker AH. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther. 2001a;4:174–181. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Reynolds PN, Brosnan MJ, White SJ, Curiel DT, Dominiczak AF, Baker AH. Analysis of cell-specific promoters for viral gene therapy targeted at the vascular endothelium. Hypertension. 2001b;38:65–70. doi: 10.1161/01.hyp.38.1.65. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant Adeno-Associated Virus Serotype 9 Leads to Preferential Cardiac Transduction In Vivo. Circ Res. 2006;99:e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Pachori AS, Melo LG, Hart ML, Noiseux N, Zhang L, Morello F, Solomon SD, Stahl GL, Pratt RE, Dzau VJ. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci U S A. 2004;101:12282–12287. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachori AS, Melo LG, Zhang L, Solomon SD, Dzau VJ. Chronic recurrent myocardial ischemic injury is significantly attenuated by pre-emptive adeno-associated virus heme oxygenase-1 gene delivery. J Am Coll Cardiol. 2006;47:635–643. doi: 10.1016/j.jacc.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S, Bueler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusola K, Kunnapuu J, Vuorikoski S, Soronen J, Andre H, Pereira T, Korpisalo P, Yla-Herttuala S, Poellinger L, Alitalo K. Stabilized HIF-1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 2005;19:1365–1367. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- Pasupathy S, Homer-Vanniasinkam S. Ischaemic preconditioning protects against ischaemia/reperfusion injury: emerging concepts. Eur J Vasc Endovasc Surg. 2005;29:106–15. doi: 10.1016/j.ejvs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Pellinen R, Hakkarainen T, Wahlfors T, Tulikami K, Ketola A, Tenhunen A, Salonen T, Wahlfors J. Cancer cells as targets for lentivirus-mediated gene transfer and gene therapy. Int J Oncol. 2004;25:1753–1762. [PubMed] [Google Scholar]
- Pislaru S, Janssens SP, Gersh BJ, Simari RD. Defining gene transfer before expecting gene therapy: putting the horse before the cart. Circulation. 2002;106:631–636. doi: 10.1161/01.cir.0000019621.18368.b7. [DOI] [PubMed] [Google Scholar]
- Pislaru SV, Simari SD. Gene transfer for ischemic cardiovascular disease: is this the end of the beginning or the beginning of the end? Nat Clin Pract Cardiovasc Med. 2005;2:138–144. doi: 10.1038/ncpcardio0136. [DOI] [PubMed] [Google Scholar]
- Post MJ, Sato K, Murakami M, Bao J, Tirziu D, Pearlman JD, Simons M. Adenoviral PR39 improves blood flow and myocar-dial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am J Physiol Regul Integr Comp Physiol. 2006;290:R494–500. doi: 10.1152/ajpregu.00460.2005. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Dormandy J, Simons M, Morishita R, Annex BH. Therapeutic angiogenesis for critical limb ischemia: design of the hepatocyte growth factor therapeutic angiogenesis clinical trial. Vasc Med. 2004;9:193–198. doi: 10.1191/1358863x04vm557oa. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Bowles DE, Faust SM, Ledford JG, Cunningham SE, Samulski RJ. Cross-dressing the virion: the transcapsidation of adeno-associated virus serotypes functionally defines subgroups. J Virol. 2004;78:4421–4432. doi: 10.1128/JVI.78.9.4421-4432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read ML, Spice R, Parker AL, Mir S, Logan A. 12th Annual congress of the European society of gene therapy. Expert Opin Biol Ther. 2005;5:137–141. doi: 10.1517/14712598.5.1.137. [DOI] [PubMed] [Google Scholar]
- Recchia A, Perani L, Sartori D, Olgiati C, Mavilio F. Site-specific integration of functional transgenes into the human genome by adeno/AAV hybrid vectors. Mol Ther. 2004;10:660–670. doi: 10.1016/j.ymthe.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL, Allen MD. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics. 2000;2:117–127. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003a;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. http://circres.ahajournals.org/cgi/ijlink?linkType=ABST&journal Code=jbc&resid=274/45/32127. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, Taipale P, Kauppinen RA, Rubanyi GM, Yla-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J. 2003b;17:100–2. doi: 10.1096/fj.02-0377fje. [DOI] [PubMed] [Google Scholar]
- Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Rutledge E, Halbert C, Russell D. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes Rother than AAV type 2. J Virol. 1998;72(1):309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda T, Kasahara N, Kedes L. Lentivirus vector-mediated gene transfer to cardiomyocytes. Methods Mol Biol. 2003;219:53–70. doi: 10.1385/1-59259-350-x:53. [DOI] [PubMed] [Google Scholar]
- Salem HK, Ranjzad P, Driessen A, Appleby CE, Heagerty AM, Kingston PA. Beta-adrenoceptor blockade markedly attenuates transgene expression from cytomegalovirus promoters within the cardiovascular system. Arterioscler Thromb Vasc Biol. 2006;26:2267–2274. doi: 10.1161/01.ATV.0000239445.67579.19. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Tonn T, Dimmeler S, Zeiher AM. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat Clin Pract Cardiovasc Med. 2006;3:S23–8. doi: 10.1038/ncpcardio0441. [DOI] [PubMed] [Google Scholar]
- Schollmann C, Grugel R, Tatje D, Hoppe J, Folkman J, Marme D, Weich HA. Basic fibroblast growth factor modulates the mitogenic potency of the platelet-derived growth factor (PDGF) isoforms by specific upregulation of the PDGF alpha receptor in vascular smooth muscle cells. J Biol Chem. 1992;267:18032–18039. [PubMed] [Google Scholar]
- Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH, Isner JM. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107:1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Schultz A, Lavie L, Hochberg I, Beyar R, Stone T, Skorecki K, Lavie P, Roguin A, Levy AP. Interindividual heterogeneity in the hypoxic regulation of VEGF: significance for the development of the coronary artery collateral circulation. Circulation. 1999;100:547–52. doi: 10.1161/01.cir.100.5.547. [DOI] [PubMed] [Google Scholar]
- Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- Shi X, Fang G, Shi W. Insertional mutagenesis at positions 520 and 584 of adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors with eliminated heparin- binding ability and introduced novel tropism. Hum Gene Ther. 2006;17:353–361. doi: 10.1089/hum.2006.17.353. [DOI] [PubMed] [Google Scholar]
- Shimpo M, Kieda U, Maeda Y, Takahashi M, Miyashita H, Miaukami H, Urale M, Kume A, Takizawa T, Shibuya M, Ozawa K, Shimada K. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc Res. 2002;53:993–1001. doi: 10.1016/s0008-6363(01)00546-6. [DOI] [PubMed] [Google Scholar]
- Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3:S123–8. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang MT, Wang BW, Chang CC, Leu JG, Kuan P, Chang H. Intramyocardial injection of naked DNA encoding HIF-1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–583. doi: 10.1016/s0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- Stachler MD, Bartlett JS. Mosaic vectors comprised of modified AAV1 capsid proteins for efficient vector purification and targeting to vascular endothelial cells. Gene Ther. 2006;13:926–931. doi: 10.1038/sj.gt.3302738. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T, Alitalo K, Achen MG. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- Stamm C, Liebold A, Steinhoff G, Strunk D. Stem cell therapy for ischemic heart disease: beginning or end of the road? Cell Transplant. 2006;15:S47–56. doi: 10.3727/000000006783982313. [DOI] [PubMed] [Google Scholar]
- Strain AJ, McGowan JA, Bucher NL. Stimulation of DNA synthesis in primary cultures of adult rat hepatocytes by rat platelet-associated substance(s) In Vitro. 1982;18:108–116. doi: 10.1007/BF02796402. [DOI] [PubMed] [Google Scholar]
- Su H, Joho S, Huang Y, Barcena A, Arakawa-Hoyt J, Grossman W, Kan YW. Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc Natl Acad Sci U S A. 2004;101:16280–16285. doi: 10.1073/pnas.0407449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Huang Y, Takagawa J, Barcena A, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006;13:1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Tsurumi Y, Couffinahl T, Sahara T, Bauters C, Symes J, Ferrara N, Isner JM. Gene transfer of naked DNA encoding for three isoforms of vascular endothelial growth factor stimulates collateral development in vivo. Lab Invest. 1996;75:487–501. [PubMed] [Google Scholar]
- Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension. 2004;43:746–751. doi: 10.1161/01.HYP.0000120152.27263.87. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–1676. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Vahlhaus C, Neumann J, Luss H, Wenzelburger F, Tjan TD, Hammel D, Scheld HH, Schmitz W, Breithardt G, Wichter T. Ischemic preconditioning by unstable angina reduces the release of CK-MB following CABG and stimulates left ventricular HSP-72 protein expression. J Card Surg. 2005;20:412–419. doi: 10.1111/j.1540-8191.2005.2004107.x. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Vassalli G, Bueler H, Dudler J, von Segesser LK, Kappenberger L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int J Cardiol. 2003;90:229–238. doi: 10.1016/s0167-5273(02)00554-5. [DOI] [PubMed] [Google Scholar]
- Velasco B, Ramirez JR, Relloso M, Li C, Kumar S, Lopez-Bote JP, Perez-Barriocanal F, Lopez-Novoa JM, Cowan PJ, d'Apice AJ, Bernabeu C. Vascular gene transfer driven by endoglin and ICAM-2 endothelial-specific promoters. Gene Ther. 2001;8:897–904. doi: 10.1038/sj.gt.3301468. [DOI] [PubMed] [Google Scholar]
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2002;283:H688–694. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH. Targeted gene delivery to vascular tissue in vivo by tropism modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- Wilhide ME, Jones WK. Potential therapeutic gene for the treatment of ischemic disease: Ad2/hypoxia-inducible factor-1alpha (HIF-1)/VP16 enhances B-type natriuretic peptide gene expression via a HIF-1-responsive element. Mol Pharmacol. 2006;69:1773–1778. doi: 10.1124/mol.106.024968. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005;23:42–47. doi: 10.1016/j.tibtech.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intra-coronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Marden JJ, Fan C, Sanlioglu S, Weiss RM, Ritchie TC, Davisson RL, Engelhardt JF. Genetic redox preconditioning differentially modulates AP-1 and NF kappa B responses following cardiac ischemia/reperfusion injury and protects against necrosis and apoptosis. Mol Ther. 2003;7:341–353. doi: 10.1016/s1525-0016(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Markkanen JE, Rissanen TT. Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc Med. 2004;14:295–300. doi: 10.1016/j.tcm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Maulik N, Ho YS, Alam J, Das DK. H(mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the Heme oxygenase-1 gene. Circulation. 2001;103:1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL, Chiorini JA. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51:563–585. [PubMed] [Google Scholar]
- Zhao J, Pettigrew GJ, Thomas J, Vandenberg JI, Delriviere L, Bolton EM, Carmichael A, Martin JL, Marber MS, Lever AM. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol. 2002;97:348–358. doi: 10.1007/s00395-002-0360-0. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]


