Abstract
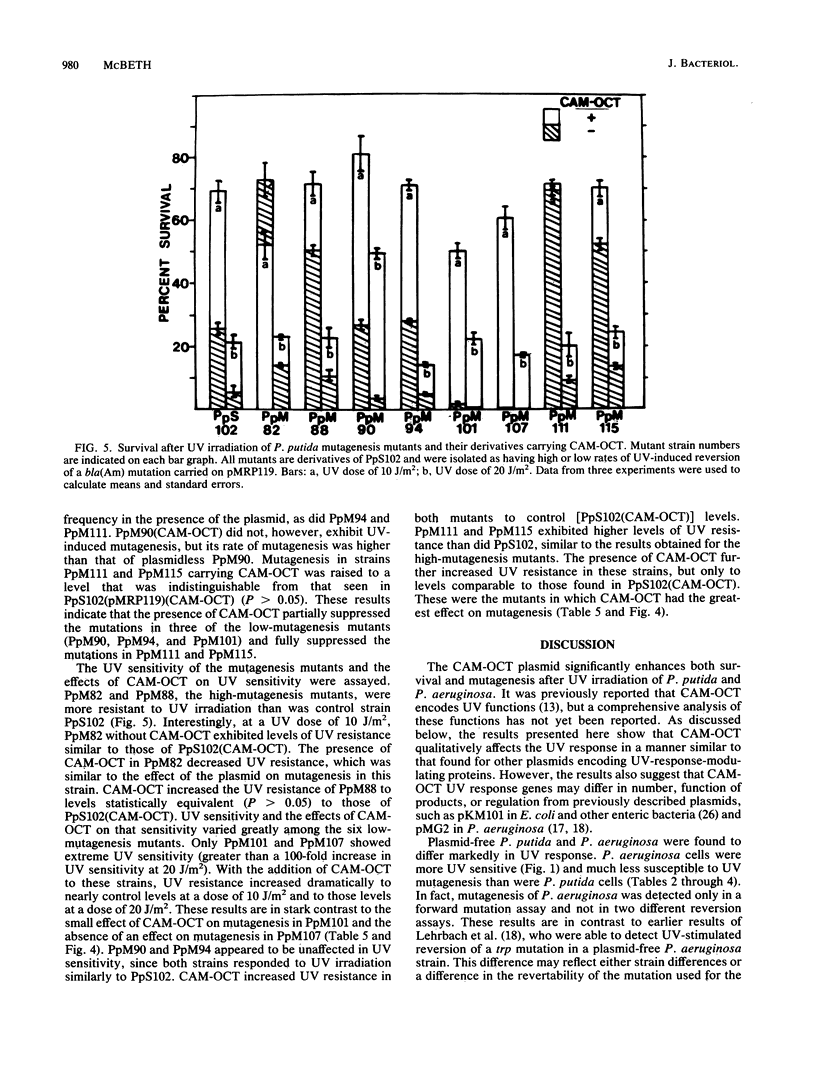
The effect of plasmid CAM-OCT on responses to UV irradiation was compared in Pseudomonas aeruginosa, in Pseudomonas putida, and in Pseudomonas putida mutants carrying mutations in UV response genes. CAM-OCT substantially increased both survival and mutagenesis in the two species. P. aeruginosa strains without CAM-OCT exhibited much higher UV sensitivity than did P. putida strains. UV-induced mutagenesis of plasmid-free P. putida was easily detected in three different assays (two reversion assays and one forward mutation assay), whereas UV mutagenesis of P. aeruginosa without CAM-OCT was seen only in the forward mutation assay. These results suggest major differences in DNA repair between the two species and highlight the presence of error-prone repair functions on CAM-OCT. A number of P. putida mutants carrying chromosomal mutations affecting either survival or mutagenesis after UV irradiation were isolated, and the effect of CAM-OCT on these mutants was determined. All mutations producing a UV-sensitive phenotype in P. putida were fully suppressed by the plasmid, whereas the plasmid had a more variable effect on mutagenesis mutations, suppressing some and producing no suppression of others. On the basis of the results reported here and results obtained by others with plasmids carrying UV response genes, it appears that CAM-OCT may differ either in regulation or in the number and functions of UV response genes encoded.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedik M., Fennewald M., Shapiro J. Transposition of a beta-lactamase locus from RP1 into Pseudomonas putida degradative plasmids. J Bacteriol. 1977 Feb;129(2):809–814. doi: 10.1128/jb.129.2.809-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Oppici M., Shapiro J., Fennewald M. Regulation of membrane peptides by the Pseudomonas plasmid alk regulon. J Bacteriol. 1979 Dec;140(3):754–762. doi: 10.1128/jb.140.3.754-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Yasbin R. E. Mutagenesis of Neisseria gonorrhoeae: absence of error-prone repair. J Bacteriol. 1984 Oct;160(1):288–293. doi: 10.1128/jb.160.1.288-293.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic fusion of incompatible plasmids in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1641–1644. doi: 10.1073/pnas.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Isolation and properties of recombination-deficient mutants of Pseudomonas aeruginosa. Mutat Res. 1974 Apr;23(1):15–23. doi: 10.1016/0027-5107(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Doisy E. A. An autobiography. Annu Rev Biochem. 1976;45:1–9. doi: 10.1146/annurev.bi.45.070176.000245. [DOI] [PubMed] [Google Scholar]
- English J. D., Vary P. S. Isolation of recombination-defective and UV-sensitive mutants of Bacillus megaterium. J Bacteriol. 1986 Jan;165(1):155–160. doi: 10.1128/jb.165.1.155-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc A. M., Sicard N., Claverys J. P., Sicard A. M. Lack of SOS repair in Streptococcus pneumoniae. Mutat Res. 1980 Apr;70(2):157–165. doi: 10.1016/0027-5107(80)90155-4. [DOI] [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. M., Ohman D. E. Transcriptional and translational analyses of recA mutant alleles in Pseudomonas aeruginosa. J Bacteriol. 1988 Apr;170(4):1637–1650. doi: 10.1128/jb.170.4.1637-1650.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L., Knobel L., Mammen P. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob Agents Chemother. 1983 Aug;24(2):168–175. doi: 10.1128/aac.24.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kung A. H., Lee B. T. The isolation and survival characterization of radiation and chemical-mutagen sensitive mutants of Pseudomonas aeruginosa. Mutat Res. 1973 Nov;20(2):175–190. doi: 10.1016/0027-5107(73)90187-5. [DOI] [PubMed] [Google Scholar]
- Lehrbach P., Kung A. H., Lee B. T., Jacoby G. A. Plasmid modification of radiation and chemical-mutagen sensitivity in Pseudomonas aeruginosa. J Gen Microbiol. 1977 Jan;98(1):167–176. doi: 10.1099/00221287-98-1-167. [DOI] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. Expression of the recA gene of Pseudomonas aeruginosa PAO is inducible by DNA-damaging agents. J Bacteriol. 1988 May;170(5):2385–2387. doi: 10.1128/jb.170.5.2385-2387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Ultraviolet light protection, enhancement of ultraviolet light mutagenesis, and mutator effect of plasmid R46 in Salmonella typhimurium. J Bacteriol. 1976 Oct;128(1):271–282. doi: 10.1128/jb.128.1.271-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Goodwin P. A. Differences in mutagenic and recombinational DNA repair in enterobacteria. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4172–4176. doi: 10.1073/pnas.82.12.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978 Sep 20;165(1):87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- Tweats D. J., Thompson M. J., Pinney R. J., Smith J. T. R factor-mediated resistance to ultraviolet light in strains of Escherichia coli deficient in known repair functions. J Gen Microbiol. 1976 Mar;93(1):103–110. doi: 10.1099/00221287-93-1-103. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Plasmid (pKM101)-mediated enhancement of repair and mutagenesis: dependence on chromosomal genes in Escherichia coli K-12. Mol Gen Genet. 1977 Mar 28;152(1):93–103. doi: 10.1007/BF00264945. [DOI] [PubMed] [Google Scholar]




