Abstract
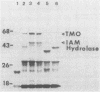
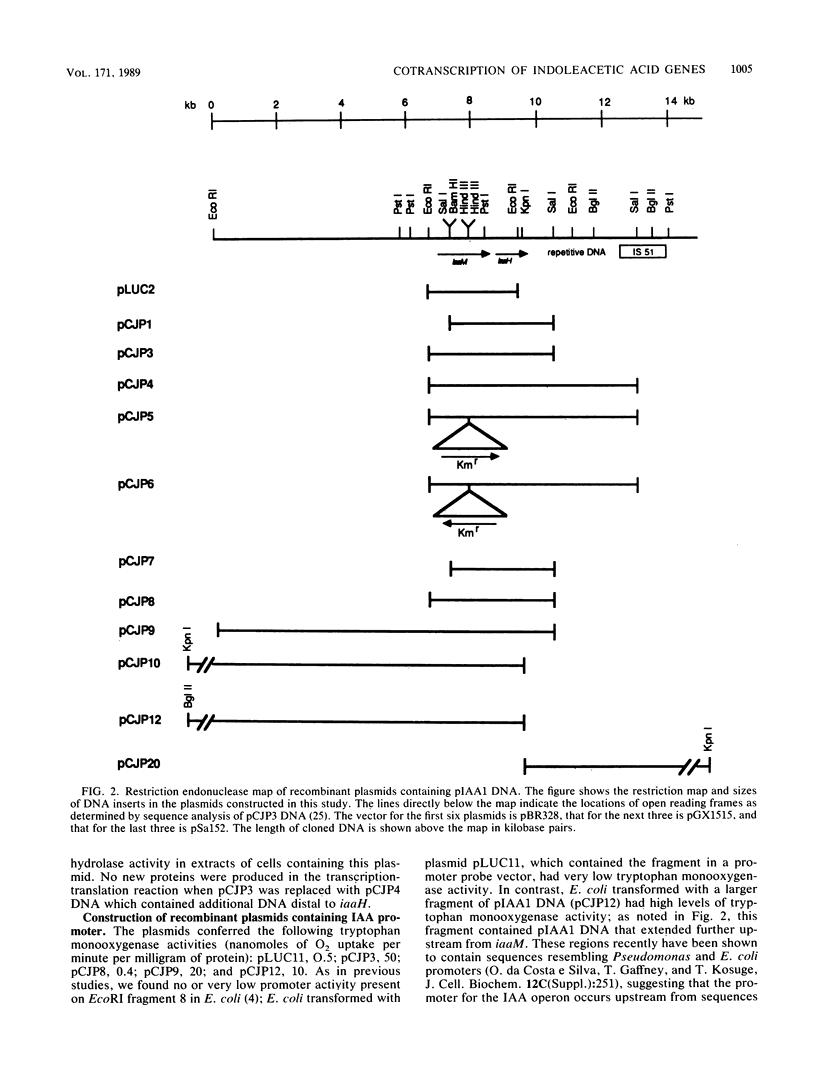
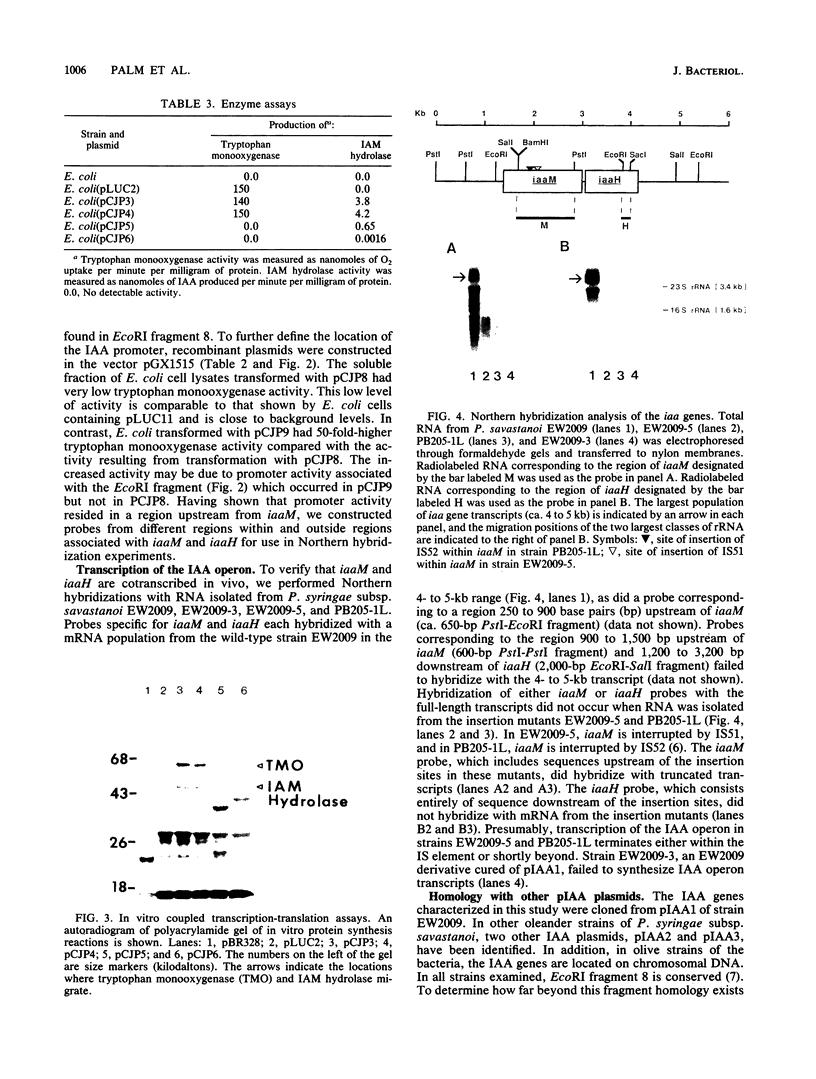
Indoleacetic acid (IAA) production by the plant pathogen Pseudomonas syringae subsp. savastanoi is essential for tumor formation on olive and oleander. The bacterium produces IAA from tryptophan in reactions catalyzed by tryptophan monooxygenase and indoleacetamide hydrolase. The genetic determinants are, respectively, iaaM and iaaH. In oleander isolates, the genes encoding the IAA biosynthetic enzymes are located on a plasmid; in olive isolates, the genes occur on the chromosome. The IAA genes from the oleander isolate strain EW2009 are located within a 4-kilobase (kb) segment of the 52-kb plasmid pIAA1. Escherichia coli strains harboring a recombinant plasmid, pCJP3, which contains this 4-kb fragment, excreted IAA into culture media, and crude cell extracts had both tryptophan monooxygenase and indoleacetamide hydrolase activity. In vitro coupled transcription-translation of pCJP3 demonstrated that this fragment coded for proteins of 62 and 47 kilodaltons which correspond to tryptophan monooxygenase and indoleacetamide hydrolase, respectively. Expression of these genes was dependent upon a vector promoter in pCJP3. However, in the absence of a vector promoter, E. coli containing recombinant plasmids with additional pIAA1 DNA in front of iaaM had high levels of tryptophan monooxygenase. Northern (RNA) hybridization experiments verified that iaaM and iaaH are cotranscribed as a portion of a ca. 4- to 5-kb transcript in vivo. Southern hybridization experiments with IAA plasmids from different oleander strains of P. syringae subsp. savastanoi revealed that all IAA plasmids contained a region of at least 10 kb of homology, with the IAA genes at one end. Repetitive DNA and a copy of IS51 were found at the end of this region of homology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Chen H. Z., Zubay G. Prokaryotic coupled transcription-translation. Methods Enzymol. 1983;101:674–690. doi: 10.1016/0076-6879(83)01047-2. [DOI] [PubMed] [Google Scholar]
- Close T. J., Tait R. C., Kado C. I. Regulation of Ti plasmid virulence genes by a chromosomal locus of Agrobacterium tumefaciens. J Bacteriol. 1985 Nov;164(2):774–781. doi: 10.1128/jb.164.2.774-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950–957. doi: 10.1128/jb.143.2.950-957.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Transposable element that causes mutations in a plant pathogenic Pseudomonas sp. J Bacteriol. 1983 Jun;154(3):1162–1167. doi: 10.1128/jb.154.3.1162-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass N. L., Kosuge T. Cloning of the gene for indoleacetic acid-lysine synthetase from Pseudomonas syringae subsp. savastanoi. J Bacteriol. 1986 May;166(2):598–603. doi: 10.1128/jb.166.2.598-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson S. W., Kosuge T. Regulation of 3-indoleacetic acid production in Pseudomonas syringae pv. savastanoi. Purification and properties of tryptophan 2-monooxygenase. J Biol Chem. 1985 May 25;260(10):6281–6287. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAGIE A. R., WILSON E. E., KOSUGE T. INDOLEACETAMIDE AS AN INTERMEDIATE IN THE SYNTHESIS OF INDOLEACETIC ACID IN PSEUDOMONAS SAVASTANOI. Science. 1963 Sep 27;141(3587):1281–1282. doi: 10.1126/science.141.3587.1281. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Lundquist R. C., Kado C. I. Genetic map of the crown gall suppressive IncW plasmid pSa. Mol Gen Genet. 1982;186(1):10–15. doi: 10.1007/BF00422905. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Yamada T., Lee P. D., Kosuge T. Insertion sequence elements of Pseudomonas savastanoi: Nucleotide sequence and homology with Agrobacterium tumefaciens transfer DNA. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8263–8267. doi: 10.1073/pnas.83.21.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Palm C. J., Brooks B., Kosuge T. Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6522–6526. doi: 10.1073/pnas.82.19.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]