Abstract
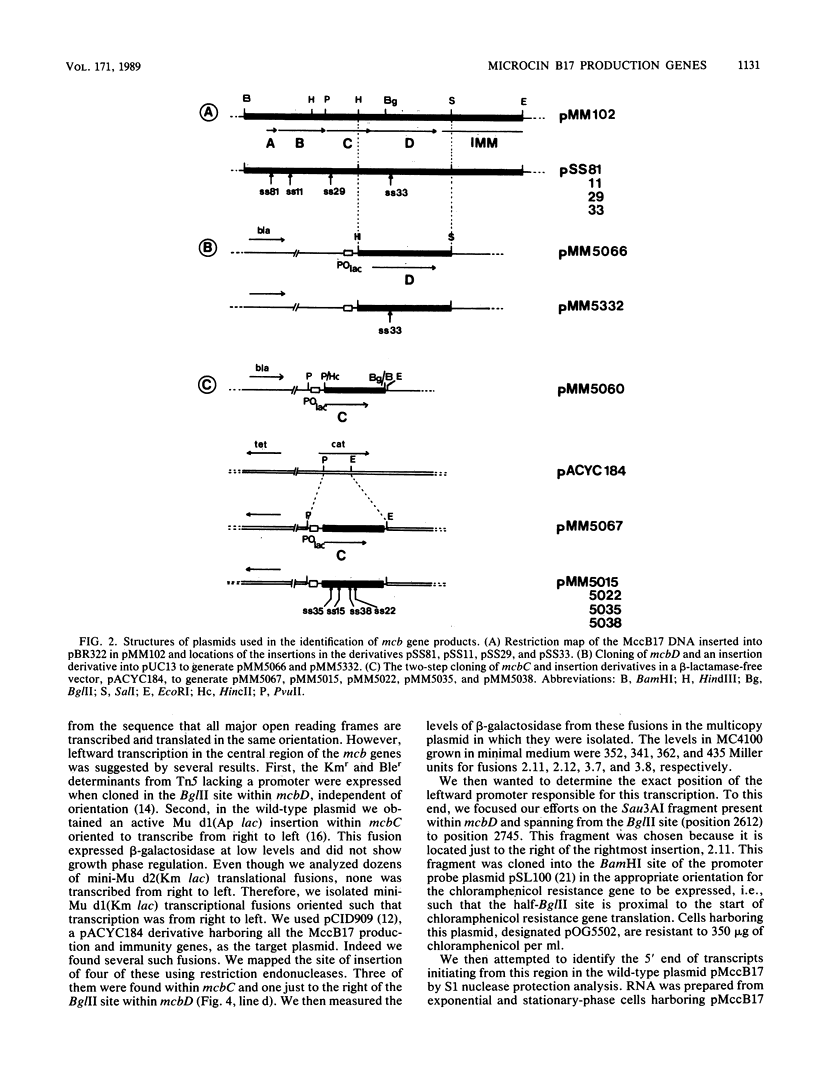
The 3.8-kilobase segment of plasmid DNA that contains the genes required for production of the DNA replication inhibitor microcin B17 was sequenced. The sequence contains four open reading frames which were shown to be translated in vivo by the construction of fusions to lacZ. The location of these open reading frames fits well with the location of the four microcin B17 production genes, mcbABCD, identified previously through genetic complementation. The products of the four genes have been identified, and the observed molecular weights of the proteins agree with those predicted from the nucleotide sequence. The transcription of these genes was studied by using fusions to lacZ and physical mapping of mRNA start sites. Three promoters were identified in this region. The major promoter for all the genes is a growth phase-regulated OmpR-dependent promoter located upstream of mcbA. A second promoter is located within mcbC and is responsible for a low-level basal expression of mcbD. A third promoter, located within mcbD, promotes transcription in the reverse direction starting within mcbD and extending through mcbC. The resulting mRNA appears to be an untranslated antisense transcript that could play a regulatory role in the expression of these genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asensio C., Pérez-Díaz J. C. A new family of low molecular weight antibiotics from enterobacteria. Biochem Biophys Res Commun. 1976 Mar 8;69(1):7–14. doi: 10.1016/s0006-291x(76)80264-1. [DOI] [PubMed] [Google Scholar]
- Baquero F., Bouanchaud D., Martinez-Perez M. C., Fernandez C. Microcin plasmids: a group of extrachromosomal elements coding for low-molecular-weight antibiotics in Escherichia coli. J Bacteriol. 1978 Aug;135(2):342–347. doi: 10.1128/jb.135.2.342-347.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Whitney P., Magasanik B. Reaction of lac-specific ribonucleic acid from Escherichia coli with lac deoxyribonucleic acid. J Biol Chem. 1974 Oct 25;249(20):6548–6555. [PubMed] [Google Scholar]
- Davagnino J., Herrero M., Furlong D., Moreno F., Kolter R. The DNA replication inhibitor microcin B17 is a forty-three-amino-acid protein containing sixty percent glycine. Proteins. 1986 Nov;1(3):230–238. doi: 10.1002/prot.340010305. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Garrido M. C., Herrero M., Kolter R., Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988 Jun;7(6):1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genilloud O., Garrido M. C., Moreno F. The transposon Tn5 carries a bleomycin-resistance determinant. Gene. 1984 Dec;32(1-2):225–233. doi: 10.1016/0378-1119(84)90050-7. [DOI] [PubMed] [Google Scholar]
- Hernández-Chico C., Herrero M., Rejas M., San Millán J. L., Moreno F. Gene ompR and regulation of microcin 17 and colicin e2 syntheses. J Bacteriol. 1982 Nov;152(2):897–900. doi: 10.1128/jb.152.2.897-900.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Chico C., San Millán J. L., Kolter R., Moreno F. Growth phase and ompR regulation of transcription of microcin B17 genes. J Bacteriol. 1986 Sep;167(3):1058–1065. doi: 10.1128/jb.167.3.1058-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M., Moreno F. Microcin B17 blocks DNA replication and induces the SOS system in Escherichia coli. J Gen Microbiol. 1986 Feb;132(2):393–402. doi: 10.1099/00221287-132-2-393. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li S. C., Squires C. L., Squires C. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell. 1984 Oct;38(3):851–860. doi: 10.1016/0092-8674(84)90280-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- San Millan J. L., Hernandez-Chico C., Pereda P., Moreno F. Cloning and mapping of the genetic determinants for microcin B17 production and immunity. J Bacteriol. 1985 Jul;163(1):275–281. doi: 10.1128/jb.163.1.275-281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millán J. L., Kolter R., Moreno F. Plasmid genes required for microcin B17 production. J Bacteriol. 1985 Sep;163(3):1016–1020. doi: 10.1128/jb.163.3.1016-1020.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Cascio M., Mielke D. L. Evaluation of methods for the prediction of membrane protein secondary structures. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9423–9427. doi: 10.1073/pnas.83.24.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]