Abstract
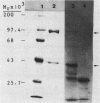
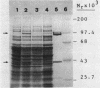
The genes that encode the two subunits of Bacillus subtilis phenylalanyl-tRNA synthetase were cloned from alpha lambda library of chromosomal B. subtilis DNA by specific complementation of a thermosensitive Escherichia coli pheS mutation. Both genes (we named them pheS and pheT, analogous to the corresponding genes of E. coli) are carried by a 6.6-kilobase-pair PstI fragment which also complements E. coli pheT mutations. This fragment directs the synthesis of two proteins identical in size to the purified alpha and beta subunits of the phenylalanyl-tRNA synthetase of B. subtilis with Mrs of 42,000 and 97,000, respectively. A recombinant shuttle plasmid carrying the genes caused 10-fold overproduction of functional phenylalanyl-tRNA synthetase in B. subtilis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg-Olivier S. A., Tarlinton D., Brown K. D. Defective regulation of the phenylalanine biosynthetic operon in mutants of the phenylalanyl-tRNA synthetase operon. J Bacteriol. 1987 May;169(5):1949–1953. doi: 10.1128/jb.169.5.1949-1953.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caillet J., Plumbridge J. A., Springer M., Vacher J., Delamarche C., Buckingham R. H., Grunberg-Manago M. Identification of clones carrying an E. coli tRNAPhe gene by suppression of phenylalanyl-tRNA synthetase thermosensitive mutants. Nucleic Acids Res. 1983 Feb 11;11(3):727–736. doi: 10.1093/nar/11.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Henning U. Tyrosine-incorporating amber suppressors in Escherichia coli K12. J Mol Biol. 1968 Apr 14;33(1):327–329. doi: 10.1016/0022-2836(68)90300-8. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruix A., Hounwanou N., Reinbolt J., Boulanger Y., Blanquet S. Purification and reversible subunit dissociation of overproduced Escherichia coli phenylalanyl-tRNA synthetase. Biochim Biophys Acta. 1983 Nov 17;741(2):244–250. doi: 10.1016/0167-4781(83)90065-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Springer M., Böck A. A specialized transducing lambda phage carrying the Escherichia coli genes for phenylalanyl-tRNA synthetase. Mol Gen Genet. 1977 Apr 29;152(3):205–210. doi: 10.1007/BF00268819. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aramori I., Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosakowski M. H., Böck A. The subunit structure of phenylalanyl-tRNA synethetase of Escherichia coli. Eur J Biochem. 1970 Jan;12(1):67–73. doi: 10.1111/j.1432-1033.1970.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Kreft J., Bernhard K., Goebel W. Recombinant plasmids capable to replication in B. subtilis and E. coli. Mol Gen Genet. 1978 Jun 1;162(1):59–67. doi: 10.1007/BF00333851. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Riva M., Waller J. P. Cloning of yeast lysyl- and phenylalanyl-tRNA synthetase genes. Biochimie. 1986 Jul-Aug;68(7-8):1001–1007. doi: 10.1016/s0300-9084(86)80043-8. [DOI] [PubMed] [Google Scholar]
- Ostroff G. R., Pène J. J. Molecular cloning with bifunctional plasmid vectors in Bacillus subtilis: isolation of a spontaneous mutant of Bacillus subtilis with enhanced transformability for Escherichia coli-propagated chimeric plasmid DNA. J Bacteriol. 1983 Nov;156(2):934–936. doi: 10.1128/jb.156.2.934-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepersberg A., Hennecke H., Engelhard M., Nass G., Böck A. Cross-reactivity of phenylalanyl-transfer ribonucleic acid ligases from different microorganisms. J Bacteriol. 1975 Dec;124(3):1482–1488. doi: 10.1128/jb.124.3.1482-1488.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Genes for the two subunits of phenylalanyl-tRNA synthesis of Escherichia coli are transcribed from the same promoter. J Mol Biol. 1980 Dec 25;144(4):595–600. doi: 10.1016/0022-2836(80)90341-1. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Steinberg W. Genetic location of two mutations affecting the lysyl-transfer ribonucleic acid synthetase of Bacillus subtilis. J Bacteriol. 1974 Oct;120(1):384–389. doi: 10.1128/jb.120.1.384-389.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M. EcoK restriction during in vitro packaging of coliphage lambda DNA. Gene. 1985;39(2-3):313–315. doi: 10.1016/0378-1119(85)90330-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M., Stahl M. M., Kobayashi I., Stahl F. W. Improved in vitro packaging of coliphage lambda DNA: a one-strain system free from endogenous phage. Gene. 1985;38(1-3):165–175. doi: 10.1016/0378-1119(85)90215-x. [DOI] [PubMed] [Google Scholar]
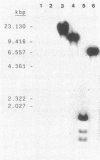
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Characterization of an E. coli mutant with a thermolabile initiation factor IF3 activity. Mol Gen Genet. 1977 Feb 28;151(1):17–26. doi: 10.1007/BF00446908. [DOI] [PubMed] [Google Scholar]
- Springer M., Mayaux J. F., Fayat G., Plumbridge J. A., Graffe M., Blanquet S., Grunberg-Manago M. Attenuation control of the Escherichia coli phenylalanyl-tRNA synthetase operon. J Mol Biol. 1985 Feb 20;181(4):467–478. doi: 10.1016/0022-2836(85)90420-6. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Anagnostopoulos C. Biochemical and genetic characterization of a temperature-sensitive, tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):6–19. doi: 10.1128/jb.105.1.6-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]