Abstract
The lipoamide dehydrogenase of the glycine decarboxylase complex was purified to homogeneity (8 U/mg) from cells of the anaerobe Eubacterium acidaminophilum that were grown on glycine. In cell extracts four radioactive protein fractions labeled with D-[2-14C]riboflavin could be detected after gel filtration, one of which coeluted with lipoamide dehydrogenase activity. The molecular mass of the native enzyme could be determined by several methods to be 68 kilodaltons, and an enzyme with a molecular mass of 34.5 kilodaltons was obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis of cell extracts separated by sodium dodecyl sulfate-polyacrylamide or linear polyacrylamide gel electrophoresis resulted in a single fluorescent band. NADPH instead of NADH was the preferred electron donor of this lipoamide dehydrogenase. This was also indicated by Michaelis constants of 0.085 mM for NADPH and 1.1 mM for NADH at constant lipoamide and enzyme concentrations. The enzyme exhibited no thioredoxin reductase, glutathione reductase, or mercuric reductase activity. Immunological cross-reactions were obtained with cell extracts of Clostridium cylindrosporum, Clostridium sporogenes, Clostridium sticklandii, and bacterium W6, but not with extracts of other glycine- or purine-utilizing anaerobic or aerobic bacteria, for which the lipoamide dehydrogenase has already been characterized.
Full text
PDF

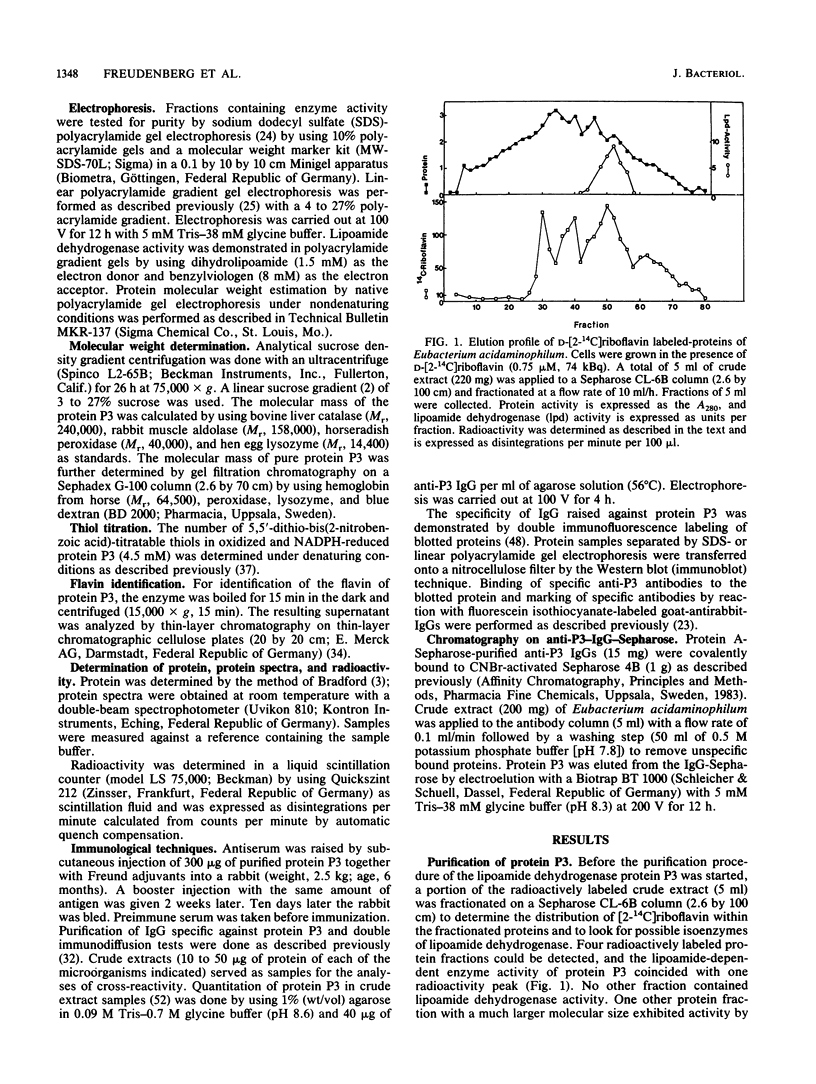
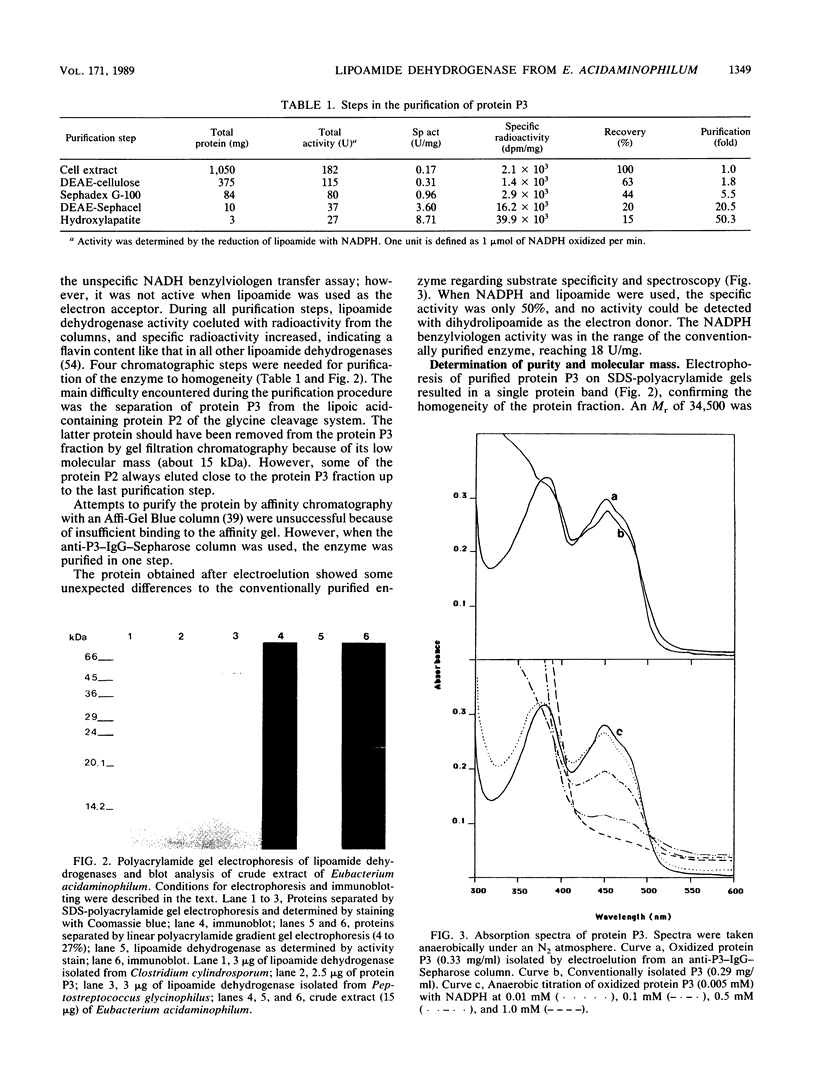





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginsky M. L., Huennekens F. M. Electron transport function of a heat-stable protein and a flavoprotein in the oxidative decarboxylation of glycine by Peptococcus glycinophilus. Biochem Biophys Res Commun. 1966 Jun 13;23(5):600–605. doi: 10.1016/0006-291x(66)90441-4. [DOI] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bresters T. W., de Abreu R. A., de Kok A., Visser J., Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. Eur J Biochem. 1975 Nov 15;59(2):335–345. doi: 10.1111/j.1432-1033.1975.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Browning K. S., Uhlinger D. J., Reed L. J. Nucleotide sequence for yeast dihydrolipoamide dehydrogenase. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1831–1834. doi: 10.1073/pnas.85.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Raefsky-Estrin C., Pons G., Patel M. S. Rat liver mitochondria contain two immunologically distinct dihydrolipoamide dehydrogenases. Arch Biochem Biophys. 1987 Aug 1;256(2):597–605. doi: 10.1016/0003-9861(87)90617-5. [DOI] [PubMed] [Google Scholar]
- Clement-Metral J. D. Activation of ALA synthetase by reduced thioredoxin in Rhodopseudomonas spheroides Y. FEBS Lett. 1979 May 1;101(1):116–120. doi: 10.1016/0014-5793(79)81307-1. [DOI] [PubMed] [Google Scholar]
- Delaney R., Burns G., Sokatch J. R. Relationship of lipoamide dehydrogenases from Pseudomonas putida to other FAD-linked dehydrogenases. FEBS Lett. 1984 Mar 26;168(2):265–270. doi: 10.1016/0014-5793(84)80259-8. [DOI] [PubMed] [Google Scholar]
- Dürre P., Andreesen J. R. Selenium-dependent growth and glycine fermentation by Clostridium purinolyticum. J Gen Microbiol. 1982 Jul;128(7):1457–1466. doi: 10.1099/00221287-128-7-1457. [DOI] [PubMed] [Google Scholar]
- Fox B., Walsh C. T. Mercuric reductase. Purification and characterization of a transposon-encoded flavoprotein containing an oxidation-reduction-active disulfide. J Biol Chem. 1982 Mar 10;257(5):2498–2503. [PubMed] [Google Scholar]
- Fujiwara K., Okamura K., Motokawa Y. Hydrogen carrier protein from chicken liver: purification, characterization, and role of its prosthetic group, lipolic acid, in the glycine cleavage reaction. Arch Biochem Biophys. 1979 Oct 15;197(2):454–462. doi: 10.1016/0003-9861(79)90267-4. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Aspects of the molecular biology of lipoamide dehydrogenase. Adv Neurol. 1978;21:219–244. [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N., Finch J. T. Structure and symmetry of B. stearothermophilus pyruvate dehydrogenase multienzyme complex and implications for eucaryote evolution. Cell. 1979 May;17(1):85–93. doi: 10.1016/0092-8674(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Purification and properties of glycine decarboxylase from chicken liver mitochondria. J Biol Chem. 1980 Dec 25;255(24):11664–11670. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K., Guest J. R., Visser J. The pyruvate dehydrogenase complex of Pseudomonas aeruginosa PAO Purification, properties and characterization of mutants. J Gen Microbiol. 1980 Oct;120(2):393–402. doi: 10.1099/00221287-120-2-393. [DOI] [PubMed] [Google Scholar]
- Kenney W. C., Zakim D., Hogue P. K., Singer T. P. Multiplicity and origin of isoenzymes of lipoyl dehydrogenase. Eur J Biochem. 1972 Jul 13;28(2):253–260. doi: 10.1111/j.1432-1033.1972.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi G., Hiraga K. The mitochondrial glycine cleavage system. Unique features of the glycine decarboxylation. Mol Cell Biochem. 1982 Jun 25;45(3):137–149. doi: 10.1007/BF00230082. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. 3. A flavin-linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):297–300. [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Reactions of glycine synthesis and glycine cleavage catalyzed by extracts of Arthrobacter globiformis grown on glycine. Arch Biochem Biophys. 1969 Jul;132(2):359–369. doi: 10.1016/0003-9861(69)90377-4. [DOI] [PubMed] [Google Scholar]
- Kohring G. W., Mayer F., Mayer H. Immunoelectron microscopic localization of the restriction endonuclease EcoRI in Escherichia coli BS 5. Eur J Cell Biol. 1985 May;37:1–6. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P., THELANDER L. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES.V. PURIFICATION AND PROPERTIES OF THIOREDOXIN REDUCTASE FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3445–3452. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. C. Polyacrylamide gel-electrophoresis across a molecular sieve gradient. Nature. 1967 Jun 24;214(5095):1334–1336. doi: 10.1038/2141334a0. [DOI] [PubMed] [Google Scholar]
- Matuda S., Saheki T. Immunochemical comparison of lipoamide dehydrogenases from various sources and reactivity of various lipoamide dehydrogenases with rat heart pyruvate dehydrogenase-subcomplex. Biochem Biophys Res Commun. 1985 Jun 14;129(2):479–484. doi: 10.1016/0006-291x(85)90176-7. [DOI] [PubMed] [Google Scholar]
- McCully V., Burns G., Sokatch J. R. Resolution of branched-chain oxo acid dehydrogenase complex of Pseudomonas aeruginosa PAO. Biochem J. 1986 Feb 1;233(3):737–742. doi: 10.1042/bj2330737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E., Hippe H., Gottschalk G. Betaine: New Oxidant in the Stickland Reaction and Methanogenesis from Betaine and l-Alanine by a Clostridium sporogenes-Methanosarcina barkeri Coculture. Appl Environ Microbiol. 1983 Feb;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H. Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases. J Biol Chem. 1987 Dec 25;262(36):17313–17318. [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- Rohde M., Mayer F., Meyer O. Immunocytochemical localization of carbon monoxide oxidase in Pseudomonas carboxydovorans. The enzyme is attached to the inner aspect of the cytoplasmic membrane. J Biol Chem. 1984 Dec 10;259(23):14788–14792. [PubMed] [Google Scholar]
- Ross J., Reid G. A., Dawes I. W. The nucleotide sequence of the LPD1 gene encoding lipoamide dehydrogenase in Saccharomyces cerevisiae: comparison between eukaryotic and prokaryotic sequences for related enzymes and identification of potential upstream control sites. J Gen Microbiol. 1988 May;134(5):1131–1139. doi: 10.1099/00221287-134-5-1131. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Identification and quantitative determination of the flavin component of soluble hydrogenase from Alcaligenes eutrophus. Biochem Biophys Res Commun. 1978 Oct 16;84(3):564–571. doi: 10.1016/0006-291x(78)90743-x. [DOI] [PubMed] [Google Scholar]
- Scouten W. H., McManus I. R. Microbial lipoamide dehydrogenase. Purification and some characteristics of the enzyme derived from selected microorganisms. Biochim Biophys Acta. 1971 Feb 10;227(2):248–263. doi: 10.1016/0005-2744(71)90058-1. [DOI] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Purification and characterization of glutathione reductase encoded by a cloned and over-expressed gene in Escherichia coli. Biochem J. 1987 Aug 1;245(3):875–880. doi: 10.1042/bj2450875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames S. L., Fairlamb A. H., Cerami A., Walsh C. T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986 Jun 17;25(12):3519–3526. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Sokatch J. R., Burns G. Oxidation of glycine by Pseudomonas putida requires a specific lipoamide dehydrogenase. Arch Biochem Biophys. 1984 Feb 1;228(2):660–666. doi: 10.1016/0003-9861(84)90036-5. [DOI] [PubMed] [Google Scholar]
- Sokatch J. R., McCully V., Gebrosky J., Sokatch D. J. Isolation of a specific lipoamide dehydrogenase for a branched-chain keto acid dehydrogenase from Pseudomonas putida. J Bacteriol. 1981 Nov;148(2):639–646. doi: 10.1128/jb.148.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokatch J. R., McCully V., Roberts C. M. Purification of a branched-chain keto acid dehydrogenase from Pseudomonas putida. J Bacteriol. 1981 Nov;148(2):647–652. doi: 10.1128/jb.148.2.647-652.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent clostridial glycine reductase. Methods Enzymol. 1978;53:373–382. doi: 10.1016/s0076-6879(78)53043-7. [DOI] [PubMed] [Google Scholar]
- Stauffer L. T., Plamann M. D., Stauffer G. V. Cloning and characterization of the glycine-cleavage enzyme system of Escherichia coli. Gene. 1986;44(2-3):219–226. doi: 10.1016/0378-1119(86)90185-x. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Lewis H. M., Darlison M. G., Guest J. R. Nucleotide sequence of the lipoamide dehydrogenase gene of Escherichia coli K12. Eur J Biochem. 1983 Oct 3;135(3):519–527. doi: 10.1111/j.1432-1033.1983.tb07683.x. [DOI] [PubMed] [Google Scholar]
- Stevenson K. J., Hale G., Perham R. N. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with mono- and bifunctional arsenoxides. Biochemistry. 1978 May 30;17(11):2189–2192. doi: 10.1021/bi00604a026. [DOI] [PubMed] [Google Scholar]
- Sundquist A. R., Fahey R. C. The novel disulfide reductase bis-gamma-glutamylcystine reductase and dihydrolipoamide dehydrogenase from Halobacterium halobium: purification by immobilized-metal-ion affinity chromatography and properties of the enzymes. J Bacteriol. 1988 Aug;170(8):3459–3467. doi: 10.1128/jb.170.8.3459-3467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. D., Setlow P. Purification and characterization of a Bacillus megaterium disulfide reductase specific for disulfides containing pantethine 4',4"-diphosphate. J Bacteriol. 1983 Jan;153(1):475–484. doi: 10.1128/jb.153.1.475-484.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S., Wand A. J., Templeton D. M., Weiss P. M. Multifunctionality of lipoamide dehydrogenase promotion of electron transferase reaction. Arch Biochem Biophys. 1983 Sep;225(2):554–561. doi: 10.1016/0003-9861(83)90067-x. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Glycine decarboxylase multienzyme complex. Purification and partial characterization from pea leaf mitochondria. J Biol Chem. 1986 Feb 15;261(5):2214–2221. [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]



