Abstract
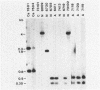
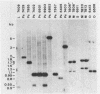
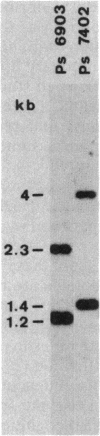
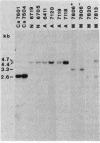
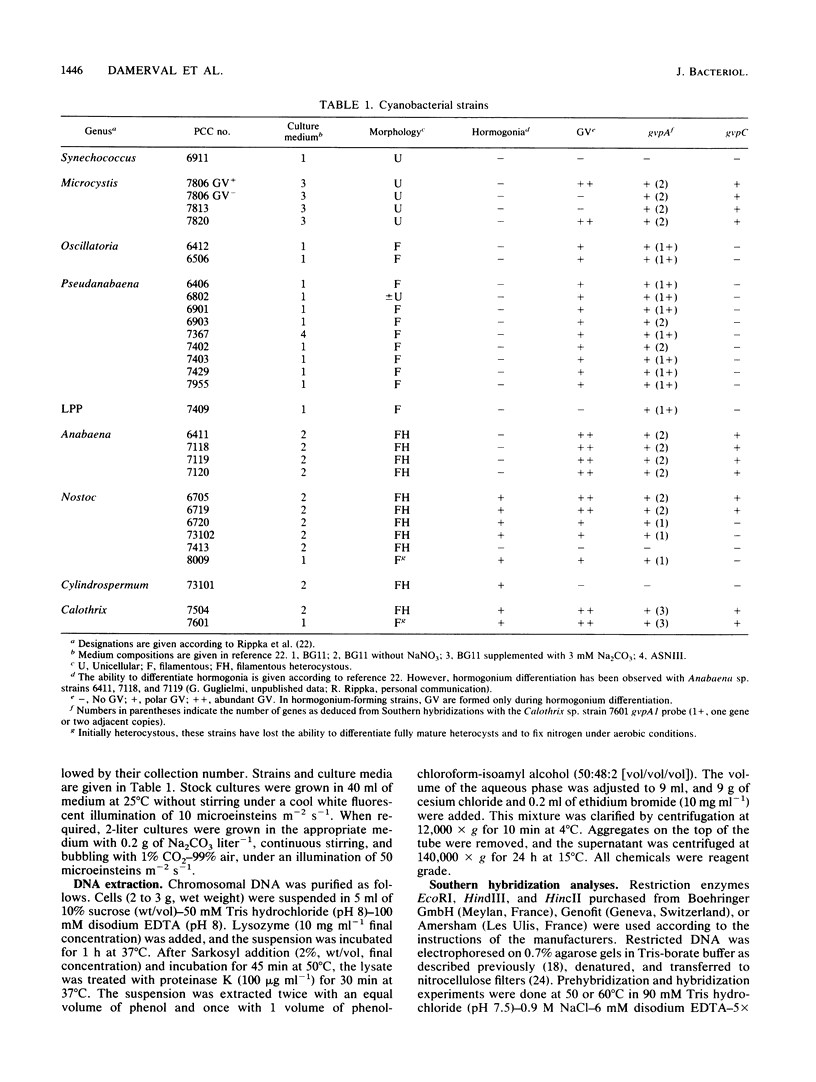
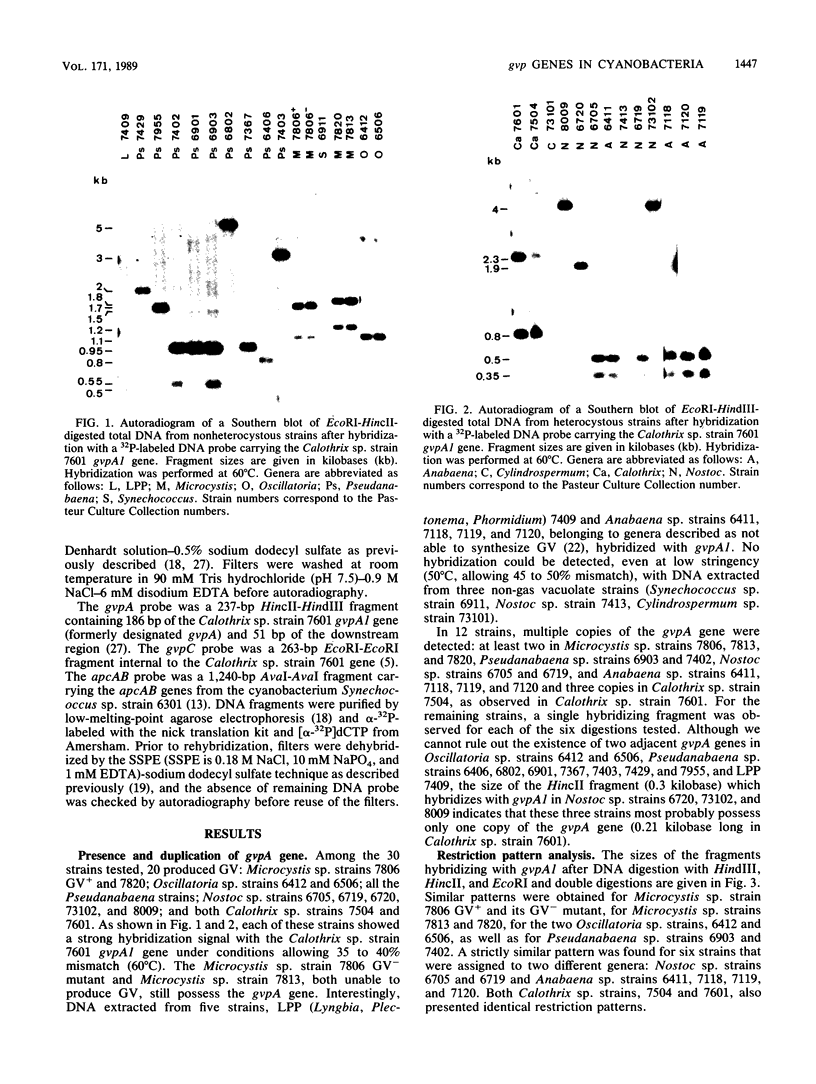
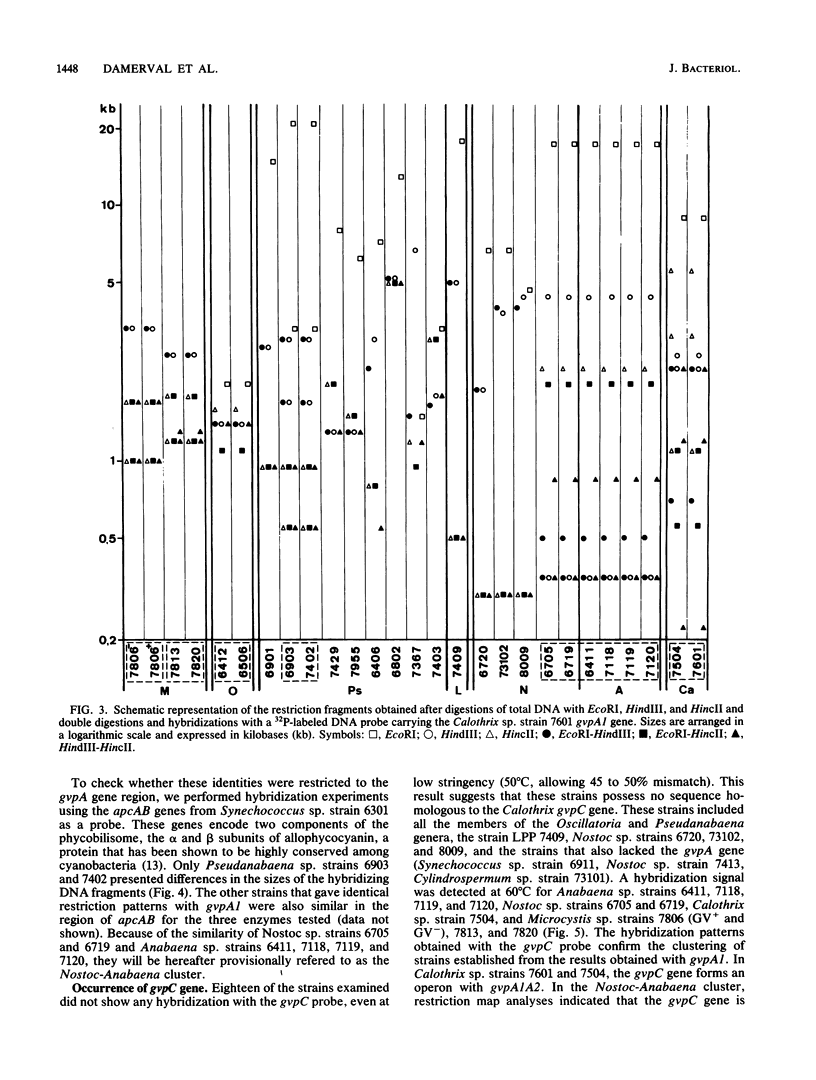
Gas vesicles (GV) are specialized cell inclusions providing many aquatic procaryotes with buoyancy. In the cyanobacterium Calothrix sp. strain PCC 7601, at least four genes are involved in GV formation. One of those, gvpA1, encodes the major structural GV protein (70 amino acids) and belongs to a multigene family (gvpA1, gvpA2, gvpD). The fourth gene, gvpC, encodes a 162-amino-acid protein, the function of which is still unclear. We used the Calothrix gvpA1 and gvpC genes as probes to perform Southern hybridization experiments with DNA extracted from various cyanobacterial strains. The gvpA gene was found in all the strains that synthesize GV, indicating that its product is an obligatory component of GV. Furthermore, it was found to occur as multiple copies in most of the strains tested. The gvpC gene was only detected in some strains able to synthesize a large amount of GV within a short period. This suggests that the gvpC gene product is a dispensable protein for GV formation and is involved in the efficiency of the assembly process. Based on the occurrence of the gvp genes and on DNA-DNA hybridization patterns, genus assignments are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki K., Tateno Y., Takahata N. Estimating evolutionary distance from restriction maps of mitochondrial DNA with arbitrary G+C content. J Mol Evol. 1981;18(1):1–8. doi: 10.1007/BF01733204. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Pfennig N. Comparative study of the structure of gas vacuoles. J Bacteriol. 1969 Nov;100(2):1049–1061. doi: 10.1128/jb.100.2.1049-1061.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszàr K., Houmard J., Damerval T., Tandeau de Marsac N. Transcriptional analysis of the cyanobacterial gvpABC operon in differentiated cells: occurrence of an antisense RNA complementary to three overlapping transcripts. Gene. 1987;60(1):29–37. doi: 10.1016/0378-1119(87)90210-1. [DOI] [PubMed] [Google Scholar]
- Damerval T., Houmard J., Guglielmi G., Csiszar K., Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Falkenberg P., Buckland B., Walsby A. E. Chemical composition of gas vesicles isolated from Anabaena flos-aquae. Arch Mikrobiol. 1972;85(4):304–309. doi: 10.1007/BF00549268. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Walsby A. E., Walker J. E. Complete amino acid sequence of cyanobacterial gas-vesicle protein indicates a 70-residue molecule that corresponds in size to the crystallographic unit cell. Biochem J. 1986 May 15;236(1):31–36. doi: 10.1042/bj2360031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Staley J. T., Lara J. C. Gas vesicle assembly in Microcyclus aquaticus. J Bacteriol. 1975 Jun;122(3):1301–1309. doi: 10.1128/jb.122.3.1301-1309.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R., Herdman M. Division patterns and cellular differentiation in cyanobacteria. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):33–39. doi: 10.1016/s0769-2609(85)80018-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Tajima F., Nei M. Biases of the estimates of DNA divergence obtained by the restriction enzyme technique. J Mol Evol. 1982;18(2):115–120. doi: 10.1007/BF01810830. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]