Abstract
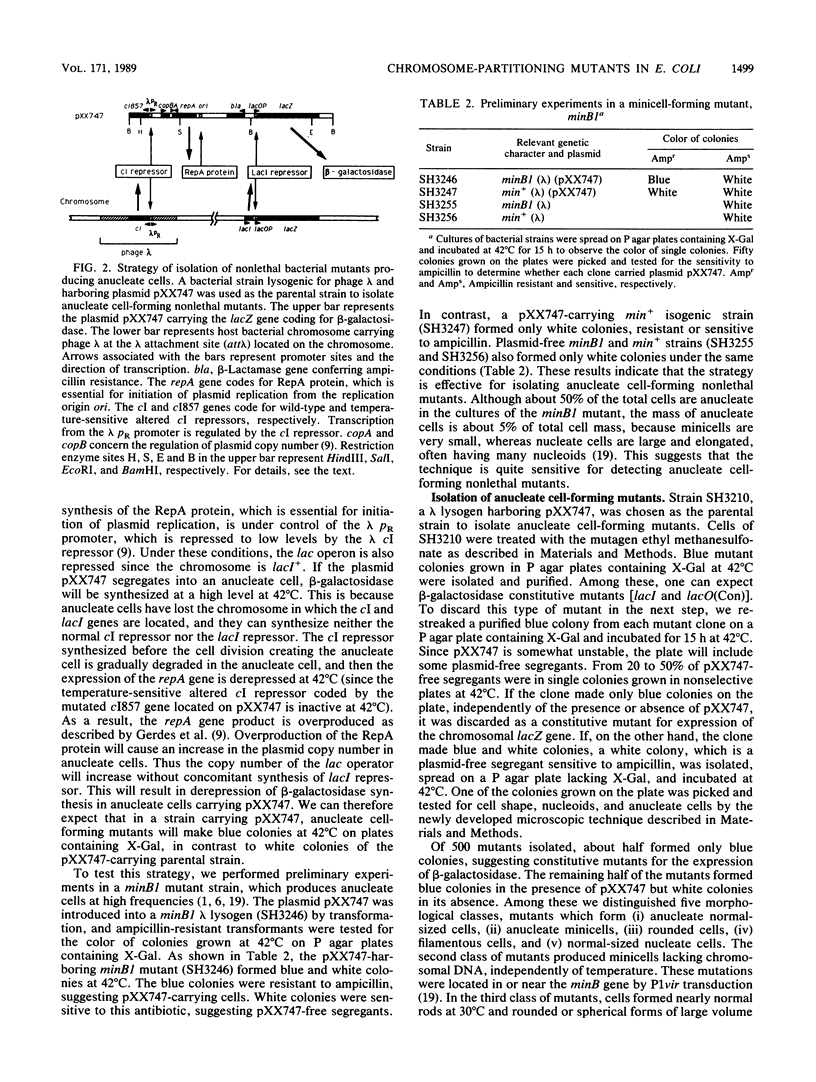
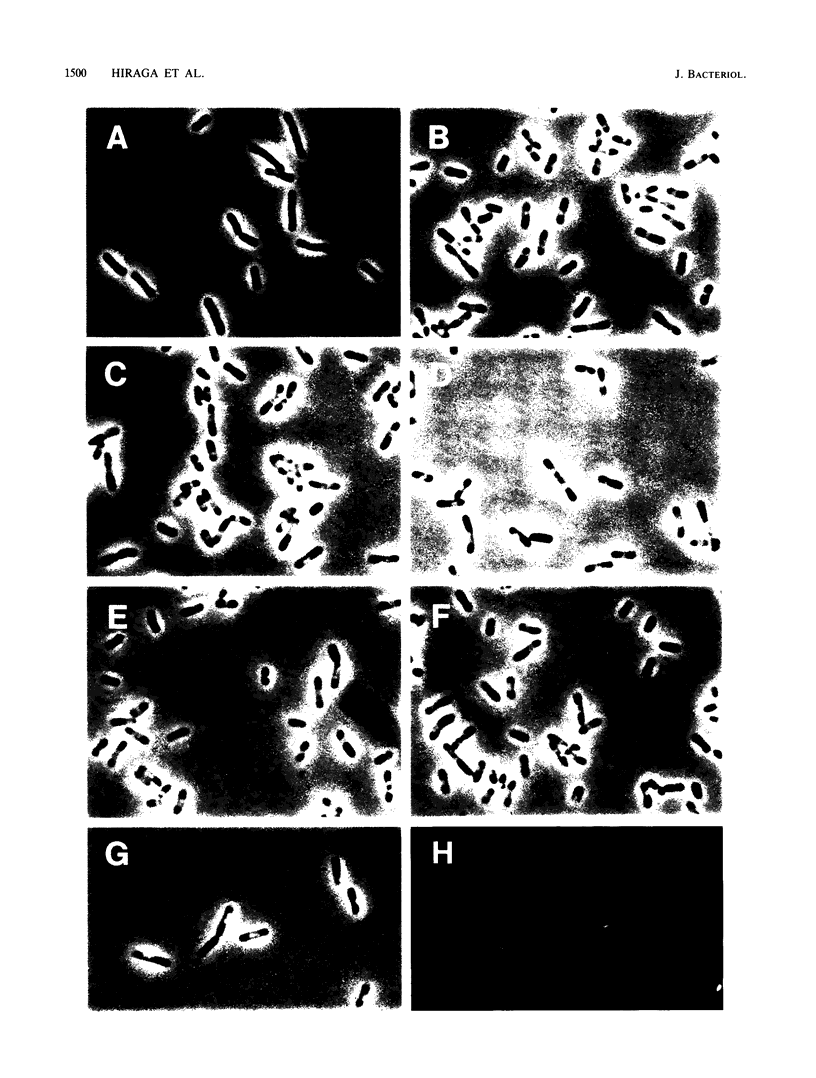
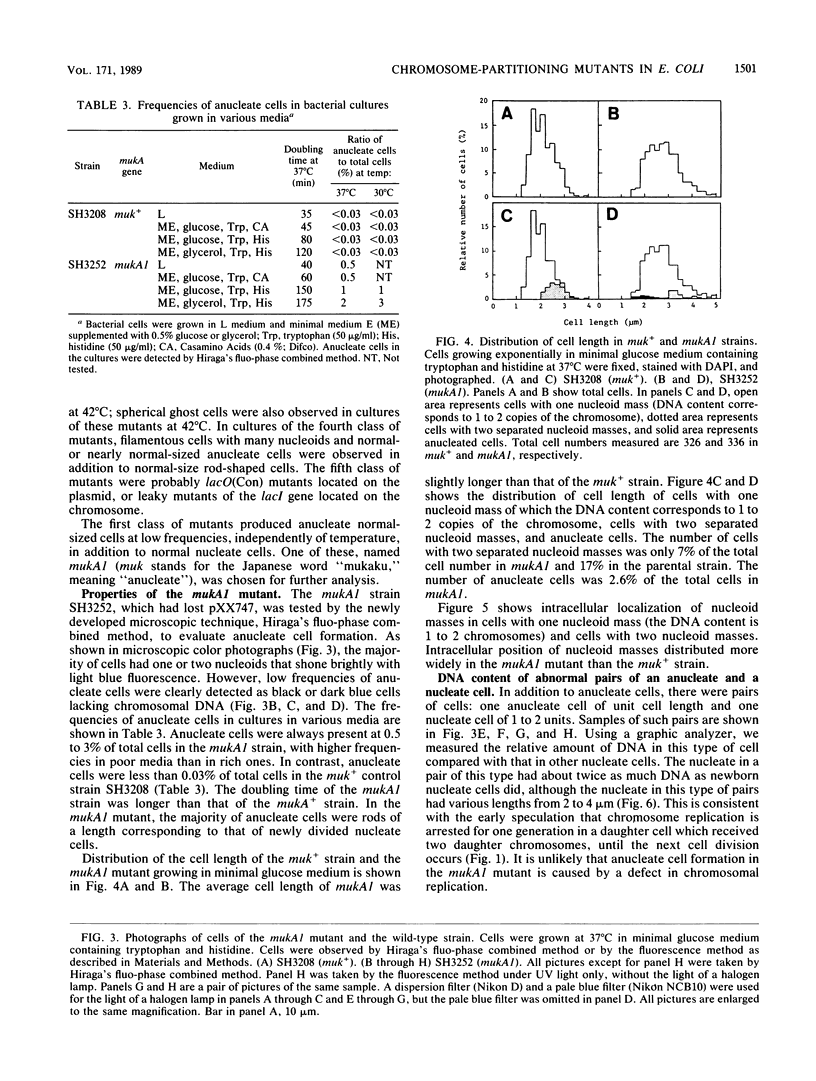
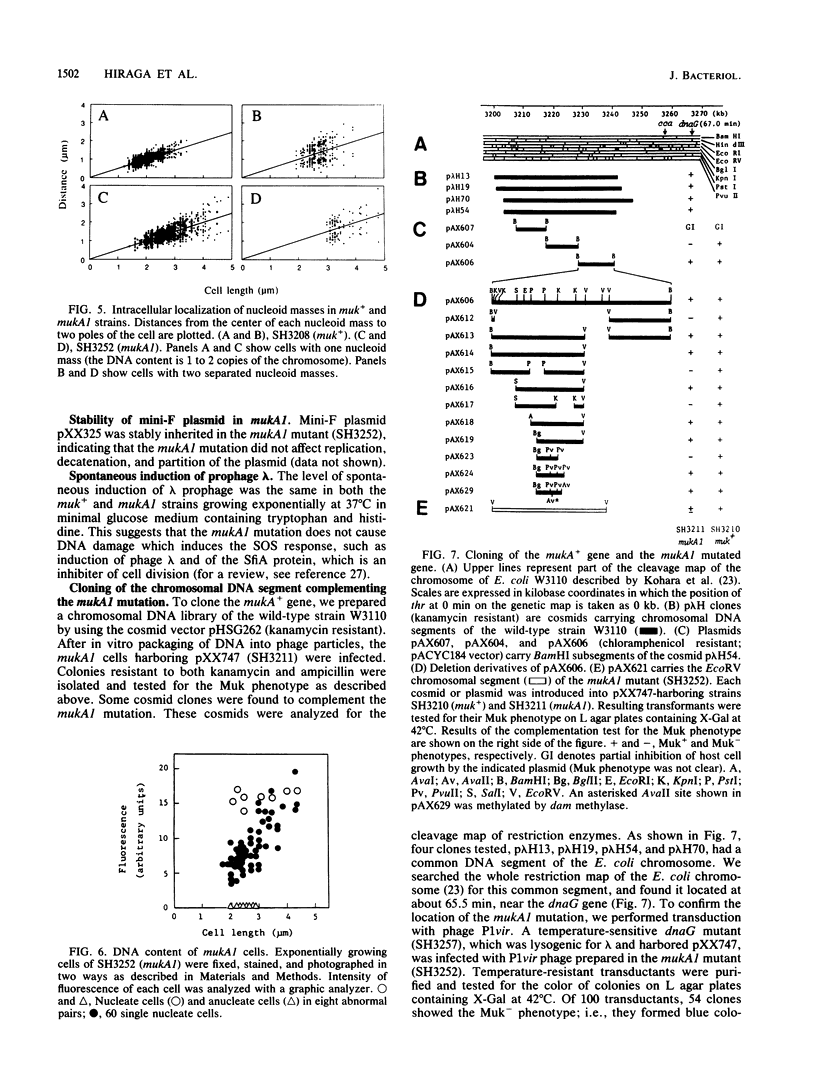
To study the chromosomal partitioning mechanism in cell division, we have isolated a novel type of Escherichia coli mutants which formed anucleate cells, by using newly developed techniques. One of them, named mukA1, is not lethal and produces normal-sized anucleate cells at a frequency of 0.5 to 3% of total cells in exponentially growing populations but does not produce filamentous cells. Results suggest that the mutant is defective in the chromosome positioning at regular intracellular positions and fails frequently to partition the replicated daughter chromosomes into both daughter cells, resulting in production of one anucleate daughter cell and one with two chromosomes. The mukA1 mutation causes pleiotropic effects: slow growth, hypersensitivity to sodium dodecyl sulfate, and tolerance to colicin E1 protein, in addition to anucleate cell formation. Cloning of the mukA gene indicates that the mukA1 mutation is recessive and that the mukA gene is identical to the tolC gene coding for an outer membrane protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady G., Jantzen H. M., Bernard H. U., Brown R., Schütz G., Hashimoto-Gotoh T. New cosmid vectors developed for eukaryotic DNA cloning. Gene. 1984 Feb;27(2):223–232. doi: 10.1016/0378-1119(84)90143-4. [DOI] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Larsen J. E., Molin S. Stable inheritance of plasmid R1 requires two different loci. J Bacteriol. 1985 Jan;161(1):292–298. doi: 10.1128/jb.161.1.292-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Misra R., Reeves P. The TolC protein of Escherichia coli K12 is synthesised in a precursor form. FEBS Lett. 1983 Jun 13;156(2):307–310. doi: 10.1016/0014-5793(83)80518-3. [DOI] [PubMed] [Google Scholar]
- Hackett J., Reeves P. Primary structure of the tolC gene that codes for an outer membrane protein of Escherichia coli K12. Nucleic Acids Res. 1983 Sep 24;11(18):6487–6495. doi: 10.1093/nar/11.18.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Mori H., Tanaka M. Mechanisms essential for stable inheritance of mini-F plasmid. Basic Life Sci. 1985;30:469–487. doi: 10.1007/978-1-4613-2447-8_34. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Normal repression in a deletion mutant lacking almost the entire operator-proximal gene of the tryptophan operon of E. coli. Nat New Biol. 1972 May 10;237(71):47–49. doi: 10.1038/newbio237047a0. [DOI] [PubMed] [Google Scholar]
- Hussain K., Begg K. J., Salmond G. P., Donachie W. D. ParD: a new gene coding for a protein required for chromosome partitioning and septum localization in Escherichia coli. Mol Microbiol. 1987 Jul;1(1):73–81. doi: 10.1111/j.1365-2958.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Hussain K., Elliott E. J., Salmond G. P. The parD- mutant of Escherichia coli also carries a gyrAam mutation. The complete sequence of gyrA. Mol Microbiol. 1987 Nov;1(3):259–273. doi: 10.1111/j.1365-2958.1987.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Hiraga S. Minicell-forming mutants of Escherichia coli: production of minicells and anucleate rods. J Bacteriol. 1988 Jul;170(7):3094–3101. doi: 10.1128/jb.170.7.3094-3101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., Ogura T., Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985 Sep;163(3):841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Nishimura Y., Yamada M., Suzuki H., Hirota Y. Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3967–3977. doi: 10.1128/jb.170.9.3967-3977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Mori H., Kondo A., Hiraga S. Partitioning of the F plasmid: overproduction of an essential protein for partition inhibits plasmid maintenance. Mol Gen Genet. 1987 Jul;208(3):365–372. doi: 10.1007/BF00328125. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H. A model of bacterial DNA segregation based upon helical geometry. J Theor Biol. 1985 Jan 7;112(1):25–39. doi: 10.1016/s0022-5193(85)80115-6. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. I. Kinetics of loss of plasmid derivatives deleted of the par region. Plasmid. 1980 Sep;4(2):215–227. doi: 10.1016/0147-619x(80)90011-6. [DOI] [PubMed] [Google Scholar]
- Norris V., Alliotte T., Jaffé A., D'Ari R. DNA replication termination in Escherichia coli parB (a dnaG allele), parA, and gyrB mutants affected in DNA distribution. J Bacteriol. 1986 Nov;168(2):494–504. doi: 10.1128/jb.168.2.494-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Orr E., Fairweather N. F., Holland I. B., Pritchard R. H. Isolation and characterisation of a strain carrying a conditional lethal mutation in the cou gene of Escherichia coli K12. Mol Gen Genet. 1979;177(1):103–112. doi: 10.1007/BF00267259. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi J. M., Karamata D. Cosegregation of cell wall and DNA in Bacillus subtilis. J Bacteriol. 1982 Dec;152(3):1231–1240. doi: 10.1128/jb.152.3.1231-1240.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]




