Abstract
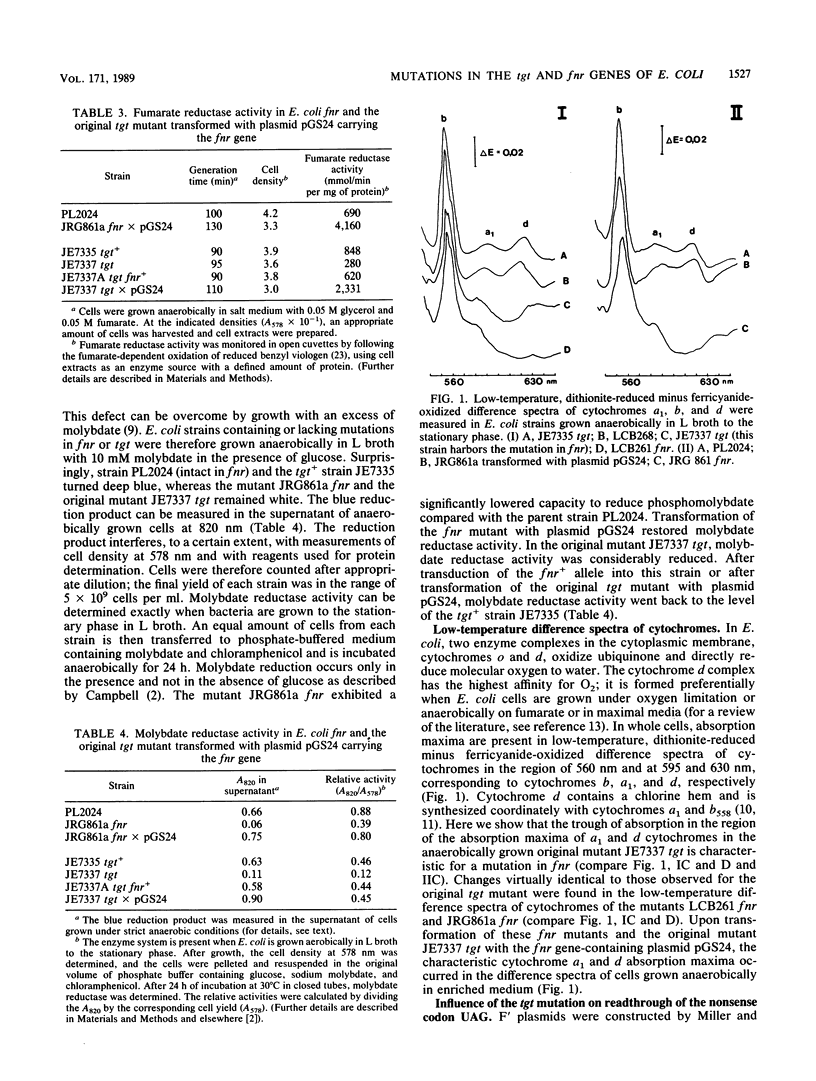
In eubacteria, the tRNA transglycosylase (Tgt) in specific tRNAs exchanges a guanine in the anticodon for 7-aminomethyl-7-deazaguanine, which is finally converted to queuosine. The tgt gene of Escherichia coli has been mapped at 9 min on the genome, and mutant pairs containing an intact or mutated tgt allele were obtained after transduction of the tgt locus by P1 bacteriophages into a genetically defined E. coli strain (S. Noguchi, Y. Nishimura, Y. Hirota, and S. Nishimura, J. Biol. Chem. 257:6544-6550, 1982). These tgt mutants grew anerobically with fumarate as an electron acceptor, while nitrate or trimethylamine N-oxide could not be reduced. Furthermore, molybdate reductase activity was almost lacking and the characteristic absorption maxima, corresponding to cytochrome a1 and the cytochrome d complex, were not detectable in low-temperature reduced-minus-oxidized difference spectra in anaerobically grown cells. Transduction of the mutated tgt locus into another E. coli recipient resulted in tgt mutants without anaerobic defects. Transformation of the original tgt mutants with an fnr gene-containing plasmid reversed the anaerobic defects. Clearly, the original tgt mutants harbor a second mutation, affecting the anaerobic regulator protein Fnr. The results suggest that fnr is involved in anaerobic control of components of the cytochrome d complex and of the redox system that transfers electrons to molybdate. F' plasmids containing a fused lacI-lacZ gene with the nonsense codon UAG at different positions in the lacI part were transferred to E. coli strains with a mutated or nonmutated tgt locus but intact in fnr. A twofold increase in the frequency of incorrect readthrough of the UAG codon, dependent on the codon context, was observed in the tgt mutant and is suggested to be caused by a tRNA(Tyr) with G in place of queuosine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouadloun F., Srichaiyo T., Isaksson L. A., Björk G. R. Influence of modification next to the anticodon in tRNA on codon context sensitivity of translational suppression and accuracy. J Bacteriol. 1986 Jun;166(3):1022–1027. doi: 10.1128/jb.166.3.1022-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M., del Campillo-Campbell A., Villaret D. B. Molybdate reduction by Escherichia coli K-12 and its chl mutants. Proc Natl Acad Sci U S A. 1985 Jan;82(1):227–231. doi: 10.1073/pnas.82.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Giudici D., Abou-Jaoudé A., Casse F., Pascal M. C. Laboratoire de Chimie Bactérienne C.N.R.S., Marsielle, France. Mol Gen Genet. 1978 Apr 6;160(2):225–229. doi: 10.1007/BF00267485. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Frey B., McCloskey J., Kersten W., Kersten H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G. N., Bray R. C., Morpeth F. F., Boxer D. H. Complexes with halide and other anions of the molybdenum centre of nitrate reductase from Escherichia coli. Biochem J. 1985 May 1;227(3):925–931. doi: 10.1042/bj2270925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Kranz J. E., Gennis R. B. Cloning the cyd gene locus coding for the cytochrome d complex of Escherichia coli. Gene. 1984 Dec;32(1-2):99–106. doi: 10.1016/0378-1119(84)90037-4. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Molybdenum effector of fumarate reductase repression and nitrate reductase induction in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3720–3725. doi: 10.1128/jb.169.8.3720-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. B., Farkas W. R., Katze J. R. Presence of queuine in Drosophila melanogaster: correlation of free pool with queuosine content of tRNA and effect of mutations in pteridine metabolism. Nucleic Acids Res. 1981 May 25;9(10):2351–2366. doi: 10.1093/nar/9.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänel G., Michelsen U., Nishimura S., Kersten H. Queuosine modification in tRNA and expression of the nitrate reductase in Escherichia coli. EMBO J. 1984 Jul;3(7):1603–1608. doi: 10.1002/j.1460-2075.1984.tb02017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten H. Anpassung des Zellstoffwechsels an Umweltänderungen: Regulation der Genexpression über Transfer-RNA und ungewöhnliche Nukleinsäurebausteine. Naturwissenschaften. 1986 Oct;73(10):593–604. doi: 10.1007/BF00368770. [DOI] [PubMed] [Google Scholar]
- Kersten H. On the biological significance of modified nucleosides in tRNA. Prog Nucleic Acid Res Mol Biol. 1984;31:59–114. doi: 10.1016/s0079-6603(08)60375-x. [DOI] [PubMed] [Google Scholar]
- Kersten H. The nutrient factor queuine: biosynthesis, occurrence in transfer RNA and function. Biofactors. 1988 Jan;1(1):27–29. [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lemire B. D., Weiner J. H. Fumarate reductase of Escherichia coli. Methods Enzymol. 1986;126:377–386. doi: 10.1016/s0076-6879(86)26038-3. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Nishimura Y., Hirota Y., Nishimura S. Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA. J Biol Chem. 1982 Jun 10;257(11):6544–6550. [PubMed] [Google Scholar]
- Okada N., Nishimura S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J Biol Chem. 1979 Apr 25;254(8):3061–3066. [PubMed] [Google Scholar]
- Okada N., Noguchi S., Kasai H., Shindo-Okada N., Ohgi T., Goto T., Nishimura S. Novel mechanism of post-transcriptional modification of tRNA. Insertion of bases of Q precursors into tRNA by a specific tRNA transglycosylase reaction. J Biol Chem. 1979 Apr 25;254(8):3067–3073. [PubMed] [Google Scholar]
- Pascal M. C., Burini J. F., Chippaux M. Regulation of the trimethylamine N-oxide (TMAO) reductase in Escherichia coli: analysis of tor::Mud1 operon fusion. Mol Gen Genet. 1984;195(1-2):351–355. doi: 10.1007/BF00332770. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Chance B. The reaction of cytochrome o in Escherichia coli K12 with oxygen. Evidence for a spectrally and kinetically distinct cytochrome o in cells from oxygen-limited cultures. J Gen Microbiol. 1981 Oct;126(2):277–287. doi: 10.1099/00221287-126-2-277. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Nucleotide sequence of the fnr gene and primary structure of the Enr protein of Escherichia coli. Nucleic Acids Res. 1982 Oct 11;10(19):6119–6130. doi: 10.1093/nar/10.19.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Tsuchiya T., Ishimoto M. Proton translocation coupled to trimethylamine N-oxide reduction in anaerobically grown Escherichia coli. J Bacteriol. 1981 Dec;148(3):762–768. doi: 10.1128/jb.148.3.762-768.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Guest J. R. Isolation and characterization of the Fnr protein, the transcriptional regulator of anaerobic electron transport in Escherichia coli. Eur J Biochem. 1985 Jan 2;146(1):193–199. doi: 10.1111/j.1432-1033.1985.tb08638.x. [DOI] [PubMed] [Google Scholar]


