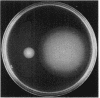
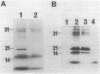
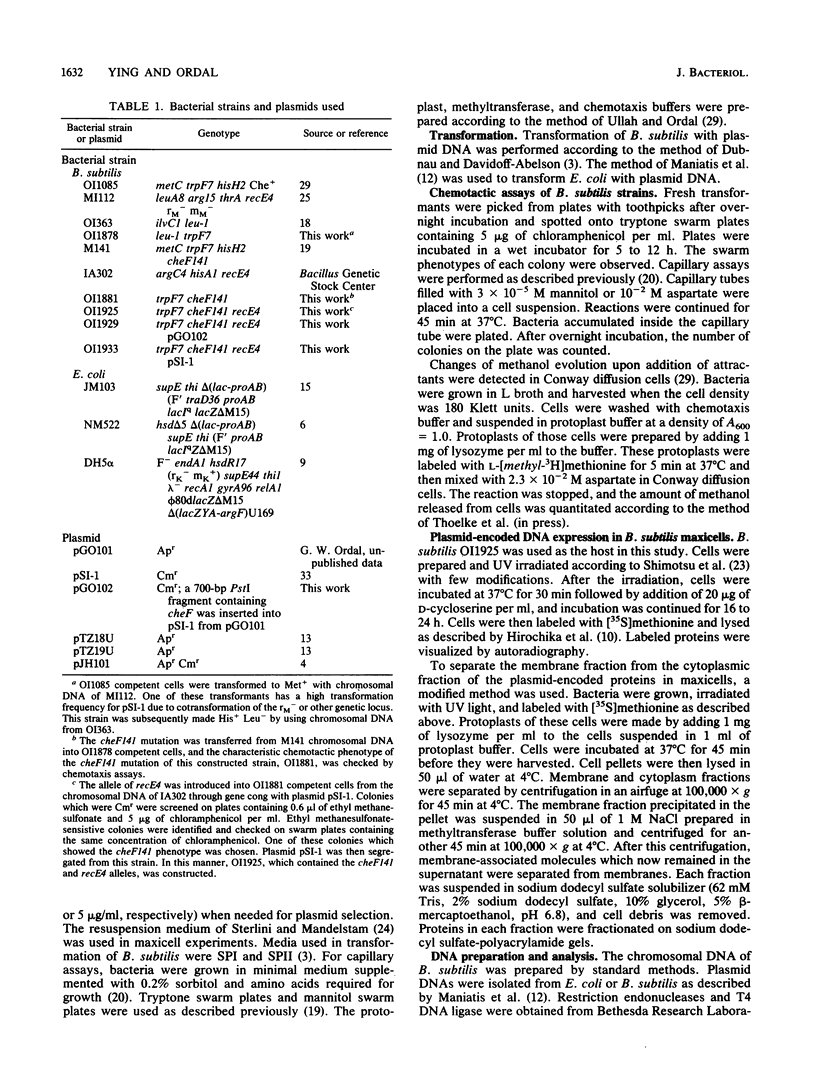
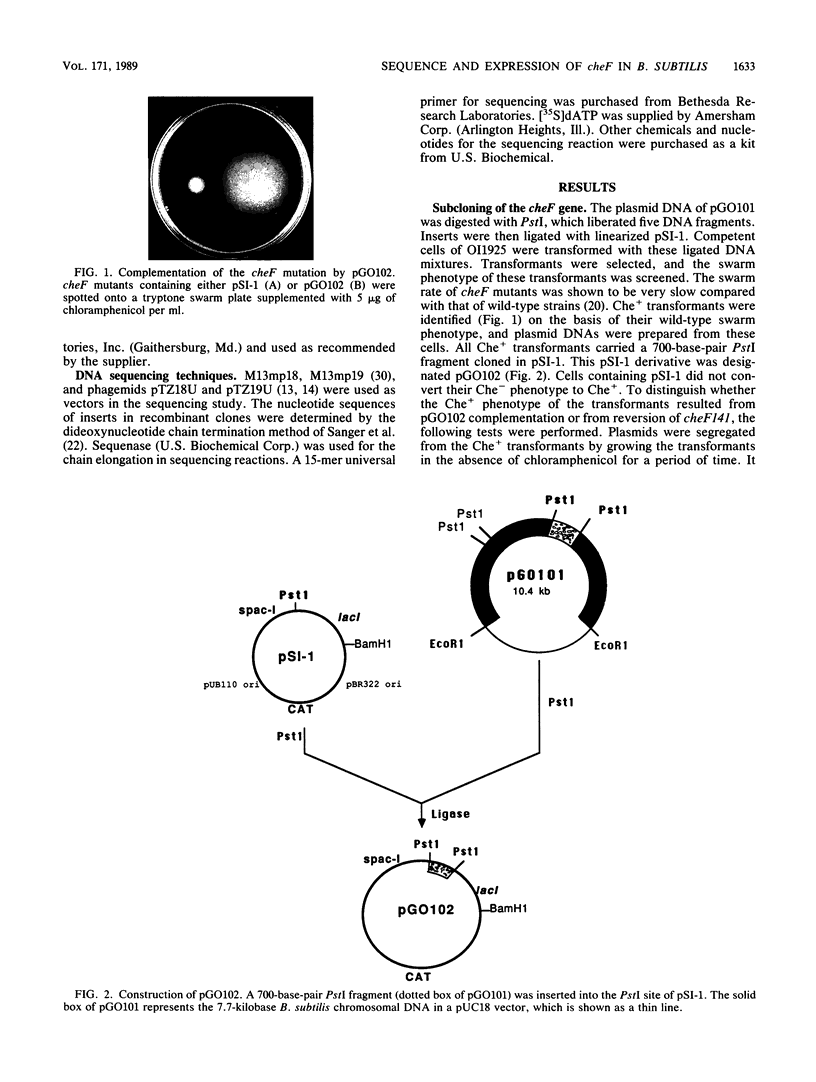
Abstract
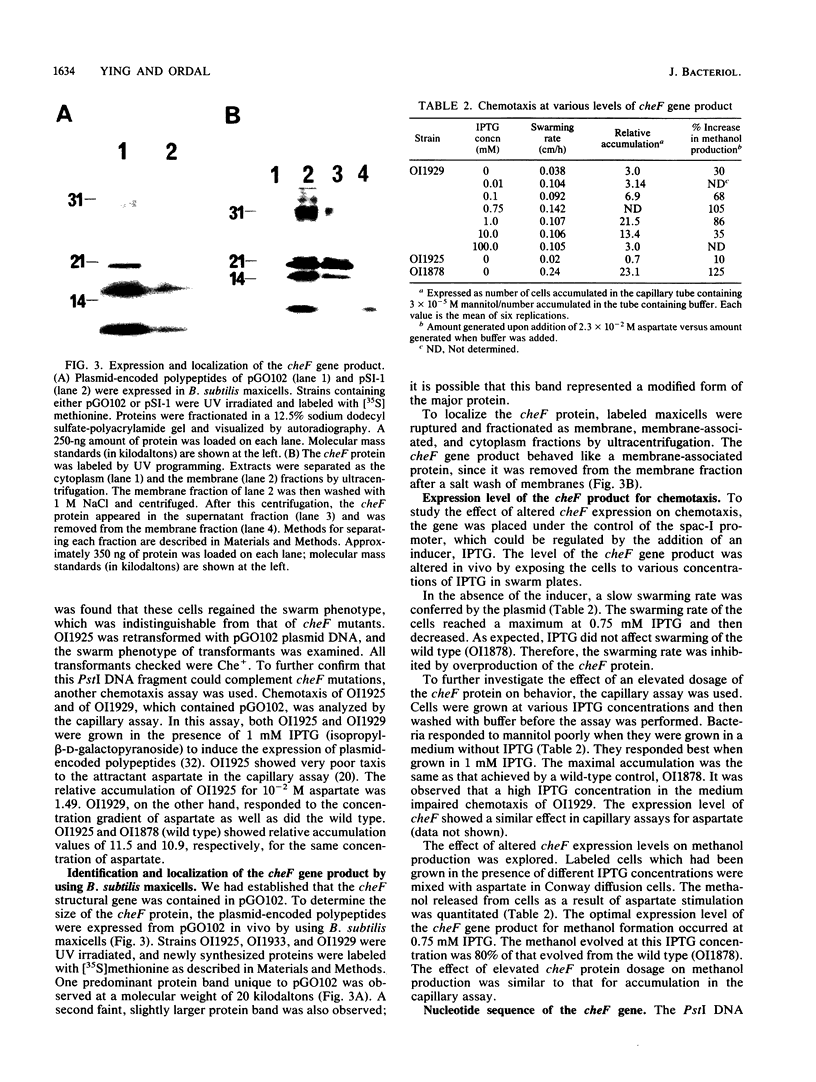
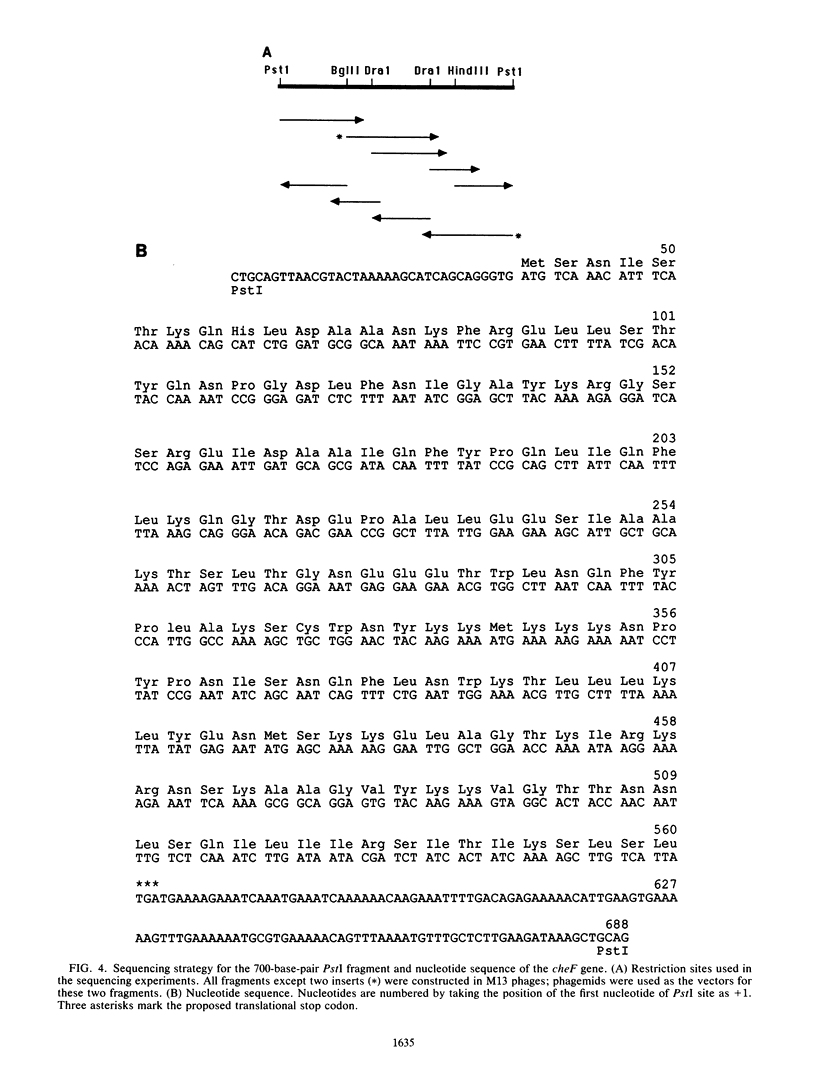
The cheF gene, which is involved in chemotaxis in Bacillus subtilis, has been cloned, expressed, and sequenced. This gene is contained in a 0.7-kilobase PstI DNA fragment that was isolated from a lambda Charon 4A B. subtilis chromosomal DNA library. This fragment was subcloned into the expression vector pSI-1 and shown to complement the cheF mutation both for chemotaxis and for methanol production in response to the addition of attractants. Plasmid-encoded DNA expression in B. subtilis maxicells indicated that a membrane-associated polypeptide of 20-kilodaltons was expressed from this 0.7-kilobase DNA. The nucleotide sequence of this DNA fragment was determined, and an open reading frame capable of encoding a putative 175-amino-acid protein (Mr 20,002) was identified. In an effort to understand the function of the cheF protein, the dosage of the cheF gene product was varied by altering the concentration of IPTG (isopropyl-beta-D-thiogalactopyranoside) during growth. In the presence of high concentrations of IPTG, chemotaxis was inhibited and methanol production was impaired.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Burgess-Cassler A., Ullah A. H., Ordal G. W. Purification and characterization of Bacillus subtilis methyl-accepting chemotaxis protein methyltransferase II. J Biol Chem. 1982 Jul 25;257(14):8412–8417. [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. J., Nettleton D. O., Ordal G. W. Purification and characterization of chemotactic methylesterase from Bacillus subtilis. Biochemistry. 1984 Feb 14;23(4):675–680. doi: 10.1021/bi00299a014. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Kobayashi Y., Kawamura F., Saito H. Cloning of sporulation gene spoOB of Bacillus subtilis and its genetic and biochemical analysis. J Bacteriol. 1981 May;146(2):494–505. doi: 10.1128/jb.146.2.494-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Simon M. I. Nucleotide sequence corresponding to five chemotaxis genes in Escherichia coli. J Bacteriol. 1986 Jan;165(1):161–166. doi: 10.1128/jb.165.1.161-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Bacterial chemotaxis: biochemistry of behavior in a single cell. Crit Rev Microbiol. 1985;12(2):95–130. doi: 10.3109/10408418509104426. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Nettleton D. O., Hoch J. A. Genetics of Bacillus subtilis chemotaxis: isolation and mapping of mutations and cloning of chemotaxis genes. J Bacteriol. 1983 Jun;154(3):1088–1097. doi: 10.1128/jb.154.3.1088-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Parker H. M., Kirby J. R. Complementation and characterization of chemotaxis mutants of Bacillus subtilis. J Bacteriol. 1985 Nov;164(2):802–810. doi: 10.1128/jb.164.2.802-810.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Kawamura F., Kobayashi Y., Saito H. Early sporulation gene spo0F: nucleotide sequence and analysis of gene product. Proc Natl Acad Sci U S A. 1983 Feb;80(3):658–662. doi: 10.1073/pnas.80.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Restriction of plasmid-mediated transformation in Bacillus subtilis 168. Mol Gen Genet. 1979 Sep;175(2):235–237. doi: 10.1007/BF00425542. [DOI] [PubMed] [Google Scholar]
- Thoelke M. S., Bedale W. A., Nettleton D. O., Ordal G. W. Evidence for an intermediate methyl-acceptor for chemotaxis in Bacillus subtilis. J Biol Chem. 1987 Feb 25;262(6):2811–2816. [PubMed] [Google Scholar]
- Thoelke M. S., Parker H. M., Ordal E. A., Ordal G. W. Rapid attractant-induced changes in methylation of methyl-accepting chemotaxis proteins in Bacillus subtilis. Biochemistry. 1988 Nov 1;27(22):8453–8457. doi: 10.1021/bi00422a024. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Toews M. L., Adler J. Methanol formation in vivo from methylated chemotaxis proteins in Escherichia coli. J Biol Chem. 1979 Mar 25;254(6):1761–1764. [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]