Abstract
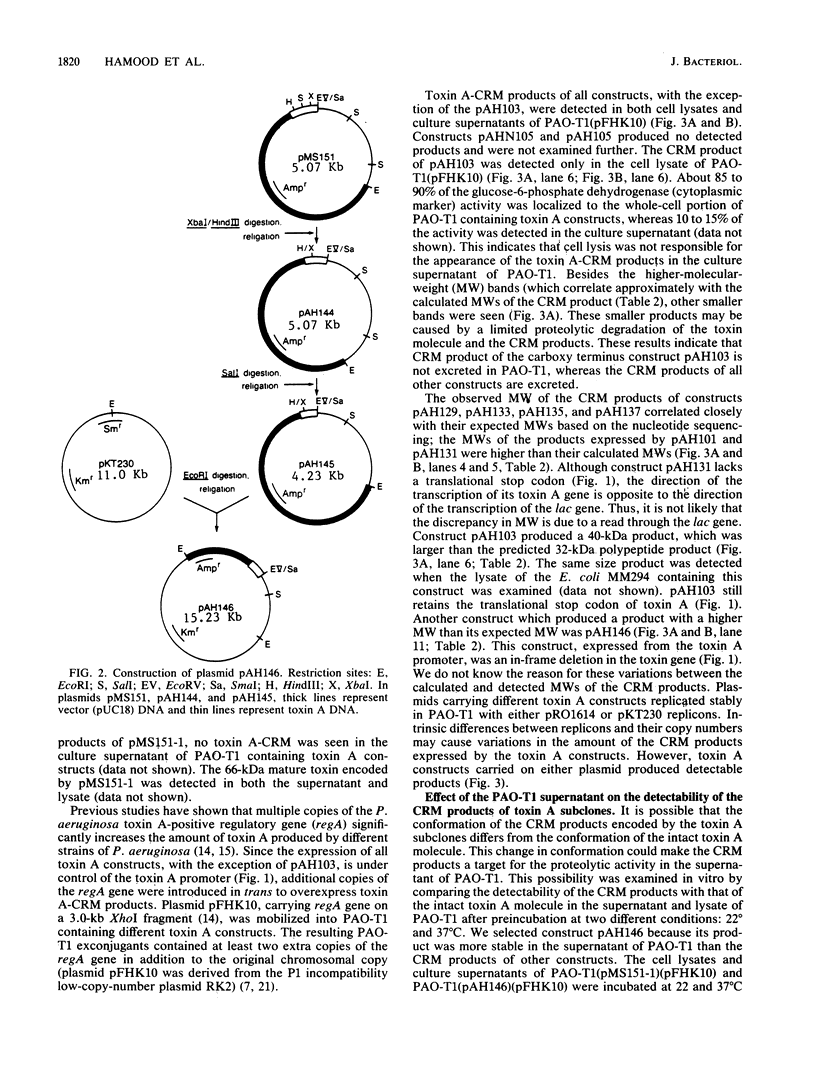
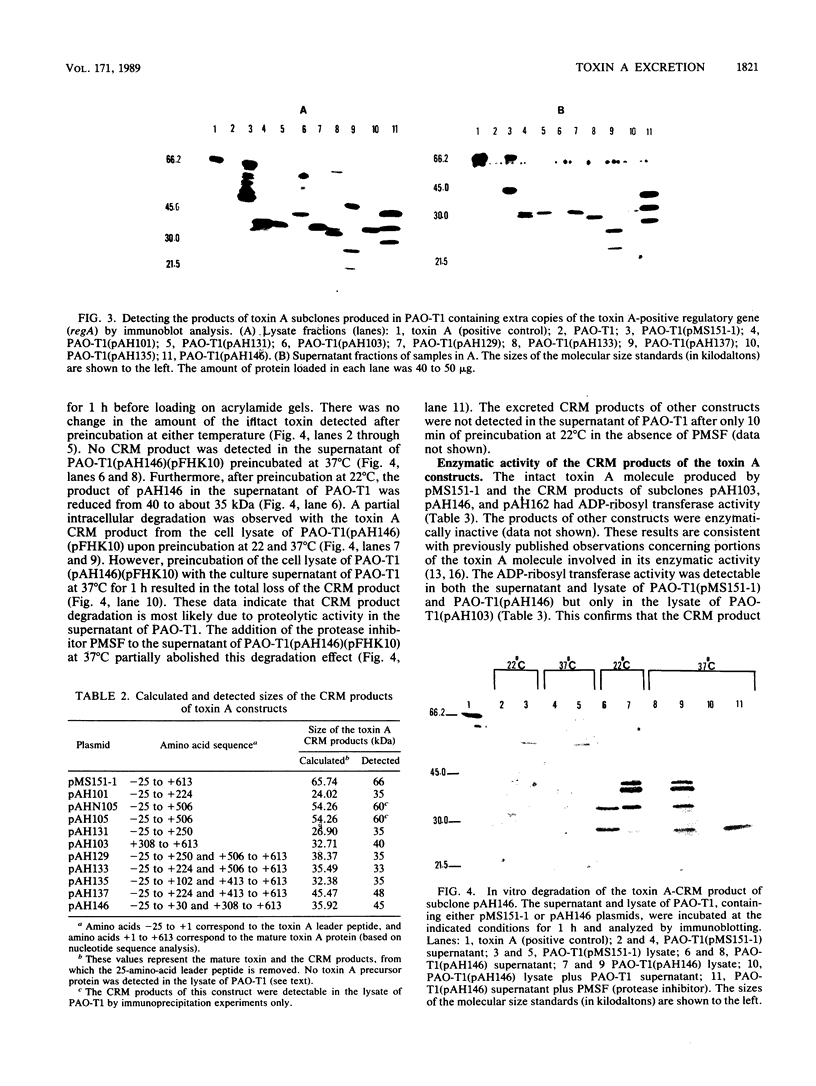
Toxin A is excreted by Pseudomonas aeruginosa as a mature 66,583-dalton protein. In this study, we used molecular cloning and deletion analysis to define specific regions of the toxin molecule involved in its excretion. Subclones that express either the amino terminus, the carboxy terminus, or toxin A molecules with internal deletions were constructed. The hypotoxigenic mutant PAO-T1 was used as a host for the expression of the toxin constructs. When overexpressed (by the presence of extra copies of the toxin A-positive regulatory gene, regA, in trans), toxin A-cross-reactive materials produced by most of these constructs were detected in the supernatant of PAO-T1. The supernatant of P. aeruginosa PAO-T1 contained proteolytic activity that degraded toxin A-derived products but not the intact toxin molecule. A single SalI intragenic deletion (coding for the leader peptide, the first 30 amino acids, and the last 305 amino acids of the toxin) resulted in a relatively stable product in the supernatant of PAO-T1. The product of the carboxy terminus construct (which codes for the last 305 amino acids of the toxin) was detected in the lysate of PAO-T1 only. The data suggest that the amino terminus region of toxin A (the leader peptide plus the first 30 amino acid of the mature protein) is sufficient for its excretion, and that a second region, amino acids 309 through 413, protects an internally truncated toxin A molecule from the proteolytic activity in the supernatant of P. aeruginosa PAO-T1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Cole S. T., Hindennach I., Henning U., Beck E., Kurz C., Schaller H. Export of a protein into the outer membrane of Escherichia coli K12. Stable incorporation of the OmpA protein requires less than 193 amino-terminal amino-acid residues. Eur J Biochem. 1982 Feb;122(1):223–231. doi: 10.1111/j.1432-1033.1982.tb05870.x. [DOI] [PubMed] [Google Scholar]
- Celis R. T. Chain-terminating mutants affecting a periplasmic binding protein involved in the active transport of arginine and ornithine in Escherichia coli. J Biol Chem. 1981 Jan 25;256(2):773–779. [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Inouye M. Nine amino acid residues at the NH2-terminal of lipoprotein are sufficient for its modification, processing, and localization in the outer membrane of Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):463–467. [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Funk C. R., Kaper J. B., Pavlovskis O. R., Galloway D. R. Cloning of a gene involved in regulation of exotoxin A expression in Pseudomonas aeruginosa. Infect Immun. 1986 Jan;51(1):37–42. doi: 10.1128/iai.51.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Frank D. W., Iglewski B. H. Molecular studies of a positive regulator of toxin A synthesis in Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:279–289. doi: 10.1159/000414353. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Ito K., Beckwith J. R. Role of the mature protein sequence of maltose-binding protein in its secretion across the E. coli cytoplasmic membrane. Cell. 1981 Jul;25(1):143–150. doi: 10.1016/0092-8674(81)90238-5. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lory S., Strom M. S., Johnson K. Expression and secretion of the cloned Pseudomonas aeruginosa exotoxin A by Escherichia coli. J Bacteriol. 1988 Feb;170(2):714–719. doi: 10.1128/jb.170.2.714-719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Burns R. P., Iglewski B. H. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980 Oct;142(4):547–555. doi: 10.1093/infdis/142.4.547. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., DeBusscher G., McCombie W. R. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1982 Apr;150(1):60–69. doi: 10.1128/jb.150.1.60-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Iglewski B. H., Pollack M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: adenosine diphosphate ribosylation of elongation factor 2. Infect Immun. 1978 Jan;19(1):29–33. doi: 10.1128/iai.19.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Richardson K., Parker C. D. Identification and characterization of Vibrio cholerae surface proteins by radioiodination. Infect Immun. 1985 Apr;48(1):87–93. doi: 10.1128/iai.48.1.87-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Mapping of export signals of Pseudomonas aeruginosa pilin with alkaline phosphatase fusions. J Bacteriol. 1987 Jul;169(7):3181–3188. doi: 10.1128/jb.169.7.3181-3188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]