Abstract
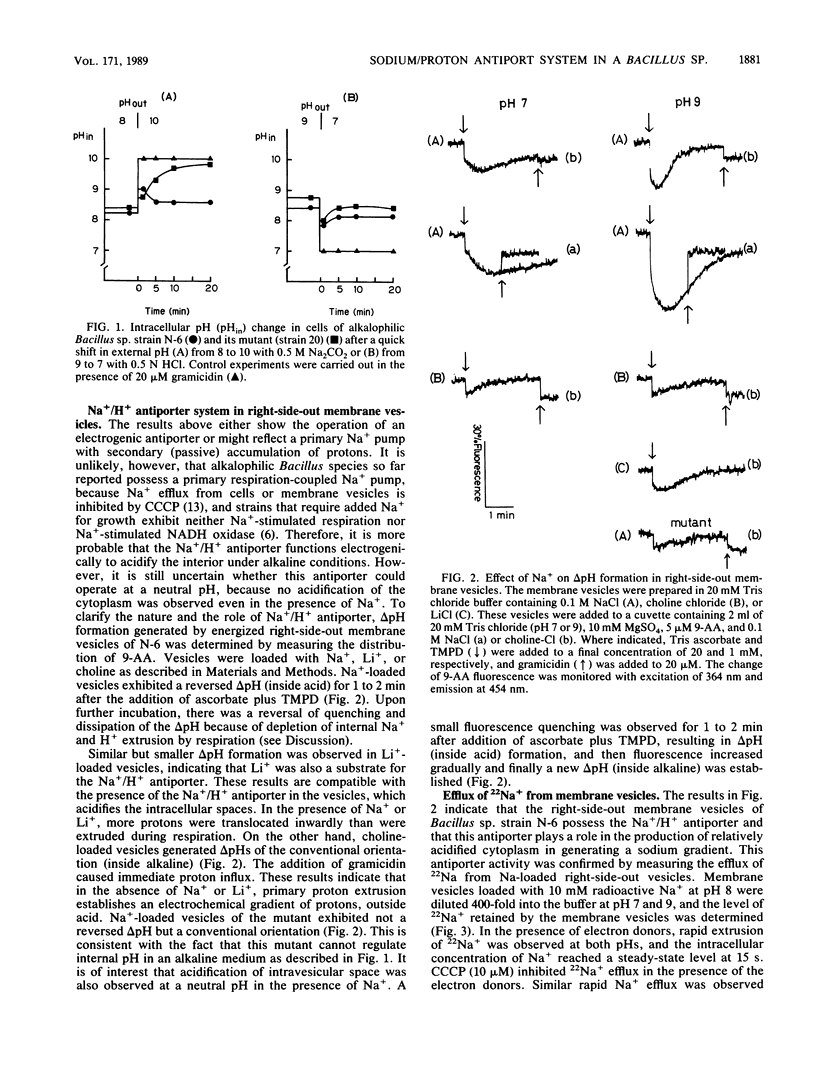
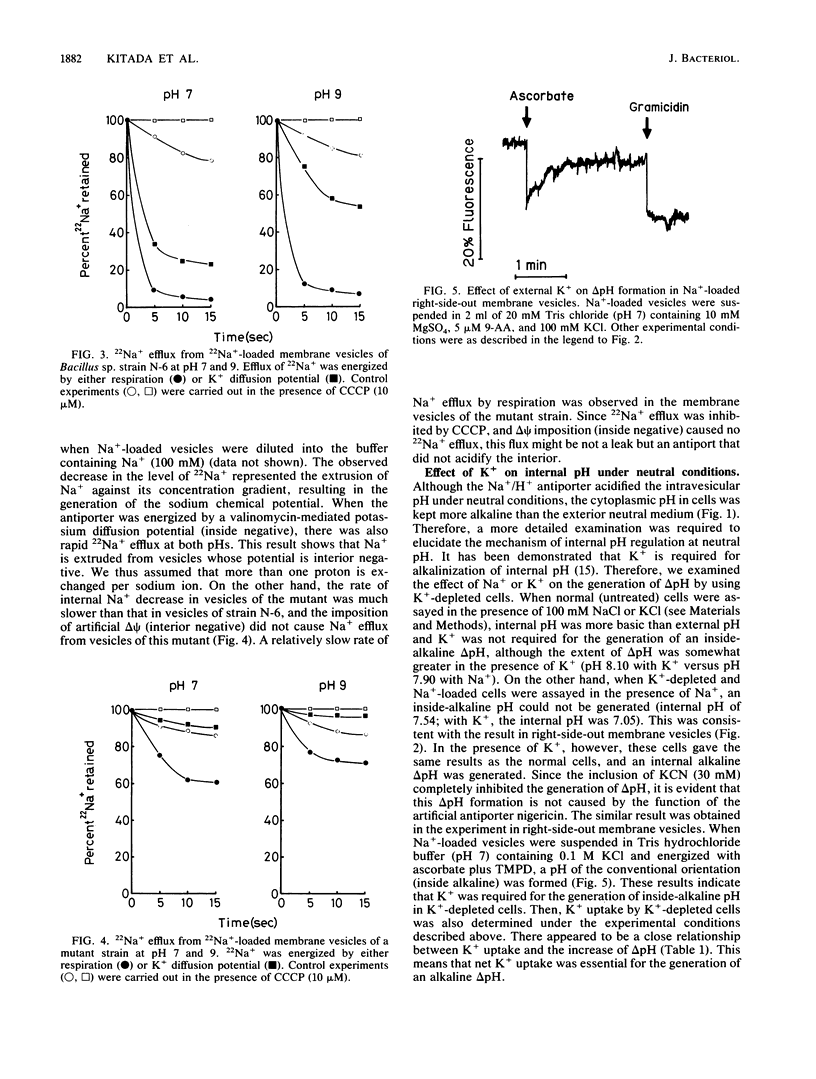
The pH homeostasis and the sodium/proton antiport system have been studied in the newly isolated alkalophilic Bacillus sp. strain N-6, which could grow on media in a pH range from 7 to 10, and in its nonalkalophilic mutant. After a quick shift in external pH from 8 to 10 by the addition of Na2CO3, the delta pH (inside acid) in the cells of strain N-6 was immediately established, and the pH homeostatic state was maintained for more than 20 min in an alkaline environment. However, under the same conditions, the pH homeostasis was not observed in the cells of nonalkalophilic mutant, and the cytoplasmic pH immediately rose to pH 10. On the other hand, the results of the rapid acidification from pH 9 to 7 showed that the internal pH was maintained as more basic than the external pH in a neutral medium in both strains. The Na+/H+ antiport system has been characterized by either the effect of Na+ on delta pH formation or 22Na+ efflux in Na+-loaded right-side-out membrane vesicles of strain N-6. Na+- or Li+-loaded vesicles exhibited a reversed delta pH (inside acid) after the addition of electron donors (ascorbate plus tetramethyl-p-phenylenediamine) at both pH 7 and 9, whereas choline-loaded vesicles generated delta pHs of the conventional orientation (inside alkaline). 22Na+ was actively extruded from 22Na+-loaded vesicles whose potential was negative at pH 7 and 9. The inclusion of carbonyl cyanide m-chlorophenylhydrazone inhibited 22Na+ efflux in the presence of electron donors. These results indicate that the Na+/H+ antiport system in this strain operates electrogenically over a range of external pHs from 7 to 10 and plays a role in pH homeostasis at the alkaline pH range. The pH homeostasis at neutral ph was studied in more detail. K+ -depleted cells showed no delta pH (acid out) in the neutral conditions in the absence of K+, whereas these cells generated a delta pH if K+ was present in the medium. This increase of internal pH was accompanied by K+ uptake from the medium. These results suggest that electrogenic K+ entry allows extrusion of H+ from cells by the primary proton pump at neutral pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbolla M. G., Rosen B. P. Energetics of sodium efflux from Escherichia coli. Arch Biochem Biophys. 1984 Feb 15;229(1):98–103. doi: 10.1016/0003-9861(84)90134-6. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Guffanti A. A., Krulwich T. A. Characterization of the Na+/H+ antiporter of alkalophilic bacilli in vivo: delta psi-dependent 22Na+ efflux from whole cells. J Bacteriol. 1983 Dec;156(3):1151–1157. doi: 10.1128/jb.156.3.1151-1157.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Ishikawa T., Hama H., Tsuda M., Tsuchiya T. Isolation and properties of a mutant of Escherichia coli possessing defective Na+/H+ antiporter. J Biol Chem. 1987 Jun 5;262(16):7443–7446. [PubMed] [Google Scholar]
- Kitada M., Guffanti A. A., Krulwich T. A. Bioenergetic properties and viability of alkalophilic Bacillus firmus RAB as a function of pH and Na+ contents of the incubation medium. J Bacteriol. 1982 Dec;152(3):1096–1104. doi: 10.1128/jb.152.3.1096-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Bioenergetic properties of alkalophilic Bacillus sp. strain C-59 on an alkaline medium containing K2CO3. J Bacteriol. 1987 Dec;169(12):5761–5765. doi: 10.1128/jb.169.12.5761-5765.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium ion-stimulated alpha-[1-14C]aminoisobutyric acid uptake in alkalophilic Bacillus species. J Bacteriol. 1977 Sep;131(3):784–788. doi: 10.1128/jb.131.3.784-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium-ion stimulated amino acid uptake in membrane vesicles of alkalophilic Bacillus no. 8-1. J Biochem. 1980 Dec;88(6):1757–1764. doi: 10.1093/oxfordjournals.jbchem.a133150. [DOI] [PubMed] [Google Scholar]
- Koyama N., Kiyomiya A., Nosoh Y. Na+-dependent uptake of amino acids by an alkalophilic Bacillus. FEBS Lett. 1976 Dec 15;72(1):77–78. doi: 10.1016/0014-5793(76)80816-2. [DOI] [PubMed] [Google Scholar]
- Kroll R. G., Booth I. R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem J. 1981 Sep 15;198(3):691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Bioenergetics of alkalophilic bacteria. J Membr Biol. 1986;89(2):113–125. doi: 10.1007/BF01869707. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Bornstein R. F., Hoffstein J. A sodium requirement for growth, solute transport, and pH homeostasis in Bacillus firmus RAB. J Biol Chem. 1982 Feb 25;257(4):1885–1889. [PubMed] [Google Scholar]
- Krulwich T. A., Hicks D. B., Seto-Young D., Guffanti A. A. The bioenergetics of alkalophilic bacilli. Crit Rev Microbiol. 1988;16(1):15–36. doi: 10.3109/10408418809104466. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tokuda H., Unemoto T. K+/H+ antiporter functions as a regulator of cytoplasmic pH in a marine bacterium, Vibrio alginolyticus. Biochim Biophys Acta. 1984 Oct 3;776(2):330–336. doi: 10.1016/0005-2736(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Plack R. H., Jr, Rosen B. P. Cation/proton antiport systems in Escherichia coli. Absence of potassium/proton antiporter activity in a pH-sensitive mutant. J Biol Chem. 1980 May 10;255(9):3824–3825. [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama S., Matsukura H., Imae Y. Relationship between Na+-dependent cytoplasmic pH homeostasis and Na+-dependent flagellar rotation and amino acid transport in alkalophilic Bacillus. FEBS Lett. 1985 Mar 25;182(2):265–268. doi: 10.1016/0014-5793(85)80312-4. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Characterization of the respiration-dependent Na+ pump in the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Sep 10;257(17):10007–10014. [PubMed] [Google Scholar]
- Zilberstein D., Padan E., Schuldiner S. A single locus in Escherichia coli governs growth in alkaline pH and on carbon sources whose transport is sodium dependent. FEBS Lett. 1980 Jul 28;116(2):177–180. doi: 10.1016/0014-5793(80)80637-5. [DOI] [PubMed] [Google Scholar]


