Abstract
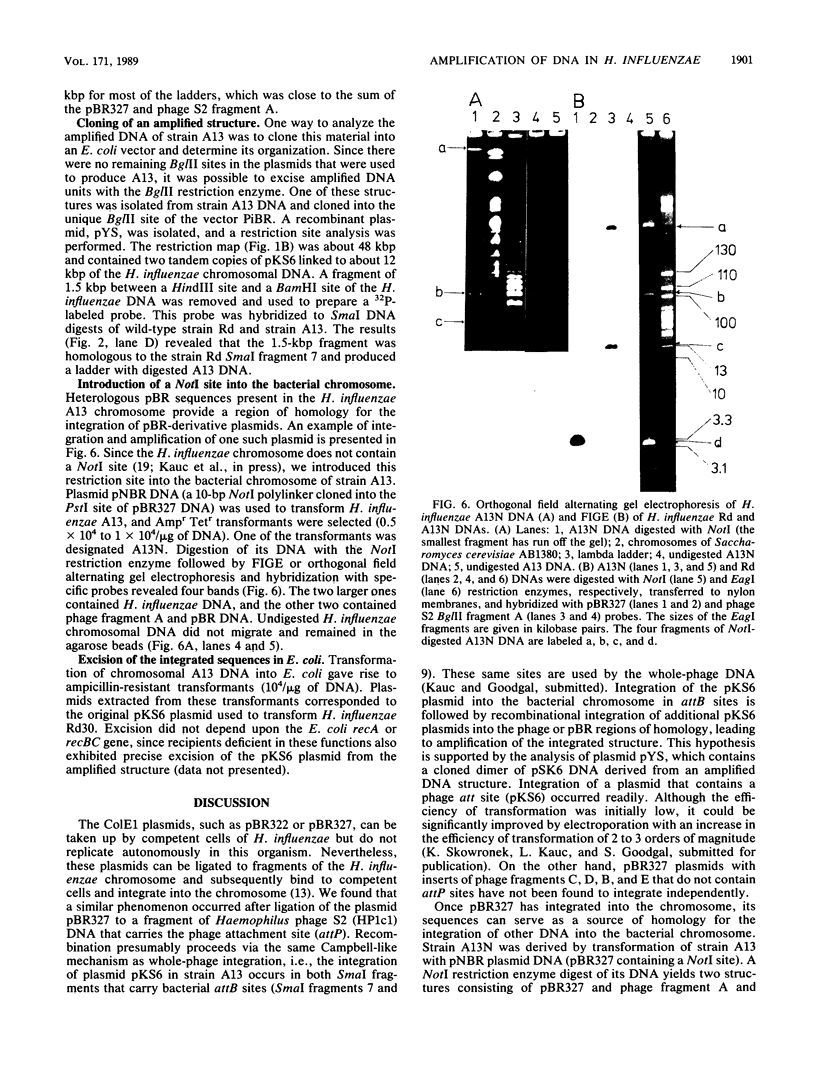
The Escherichia coli plasmids pBR322 and pBR327 can be taken up by Haemophilus influenzae but do not replicate in this organism; however, integration of pBR into the H. influenzae chromosome was achieved by ligation to a fragment of the Haemophilus phage S2 that carried a phage attachment site (attP). Once these sequences were integrated, they could serve as sites of recombination and amplification for homologous (pBR or phage) DNA. Amplification appeared to occur in one of two prophage sites (attB) present in the H. influenzae chromosome. The extent of amplification was different in different cells and reflected the ability of these sequences to undergo rearrangement leading to the formation of a DNA ladder. The ladder was obtained by treatment of DNA with restriction enzymes that cut outside of the inserted DNA, i.e., did not cut in the repeat sequence, and represented different numbers of repeat elements. Reversed-field gel electrophoresis was instrumental in resolving amplified structures. Inasmuch as single-cell isolates gave rise to the same ladder structure, it was assumed that amplification was under regulatory control and that it reproduced the same equilibrium of repeat structures. Transformation of E. coli with the amplified H. influenzae DNA resulted in precise excision and replication of the original monomeric plasmids. This excision was independent of the recA and recBC genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Galizzi A. Amplification of a chromosomal region in Bacillus subtilis. J Bacteriol. 1985 Jun;162(3):1203–1211. doi: 10.1128/jb.162.3.1203-1211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Borst P., Van der Bliek A. M., Van der Velde-Koerts T., Hes E. Structure of amplified DNA, analyzed by pulsed field gradient gel electrophoresis. J Cell Biochem. 1987 Aug;34(4):247–258. doi: 10.1002/jcb.240340404. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Smith C. L., Mathew M. K. Pulsed-field gel electrophoresis of very large DNA molecules. Annu Rev Biophys Biophys Chem. 1988;17:287–304. doi: 10.1146/annurev.bb.17.060188.001443. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. W., Piekarowicz A. Host specificity of DNA in Haemophilus influenzae: restriction and modification in strain Rd. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1610–1617. doi: 10.1016/0006-291x(72)90793-0. [DOI] [PubMed] [Google Scholar]
- Grundy F. J., Plaut A., Wright A. Haemophilus influenzae immunoglobulin A1 protease genes: cloning by plasmid integration-excision, comparative analyses, and localization of secretion determinants. J Bacteriol. 1987 Oct;169(10):4442–4450. doi: 10.1128/jb.169.10.4442-4450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N. I., Koshland D. E., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Ehrlich S. D. Repeated DNA sequences recombine 1,000 times more frequently in a plasmid than in the chromosome of Bacillus subtilis. Basic Life Sci. 1985;30:93–103. doi: 10.1007/978-1-4613-2447-8_9. [DOI] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Michalka J., Goodgal S. H. Genetic and physical map of the chromosome of Hemophilus influenzae. J Mol Biol. 1969 Oct 28;45(2):407–421. doi: 10.1016/0022-2836(69)90115-6. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Ehrlich S. D. In vitro genetic labeling of Bacillus subtilis cryptic plasmid pHV400. Plasmid. 1979 Jan;2(1):48–58. doi: 10.1016/0147-619x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Passananti C., Davies B., Ford M., Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification. EMBO J. 1987 Jun;6(6):1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronek K., Piekarowicz A., Kauc L. Comparison of HP1c1 and S2 phages of Haemophilus influenzae. Acta Microbiol Pol. 1986;35(3-4):227–232. [PubMed] [Google Scholar]
- Spies T., Laufs R. Circularized copies of amplifiable resistance genes from Haemophilus influenzae plasmids. J Bacteriol. 1983 Dec;156(3):1263–1267. doi: 10.1128/jb.156.3.1263-1267.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies T., Laufs R., Riess F. C. Amplification of resistance genes in Haemophilus influenzae plasmids. J Bacteriol. 1983 Aug;155(2):839–846. doi: 10.1128/jb.155.2.839-846.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Fitzmaurice W. P., Scocca J. J. Integration of the bacteriophage HP1c1 genome into the Haemophilus influenzae Rd chromosome in the lysogenic state. J Bacteriol. 1986 Jan;165(1):297–300. doi: 10.1128/jb.165.1.297-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M., Cullum J. A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett. 1987 Feb 9;212(1):10–14. doi: 10.1016/0014-5793(87)81547-8. [DOI] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]
- Young M. The mechanism of insertion of a segment of heterologous DNA into the chromosome of Bacillus subtilis. J Gen Microbiol. 1983 May;129(5):1497–1512. doi: 10.1099/00221287-129-5-1497. [DOI] [PubMed] [Google Scholar]







