Abstract
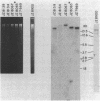
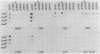
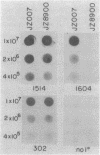
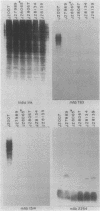
Monoclonal antibodies (MAbs) with affinities for molecules on the cell surface of the procaryote Myxococcus xanthus were used in a screening strategy for the isolation of mutants lacking particular cell surface molecules. From a large library of independent mutants created by Tn5 transposon mutagenesis, mutants were isolated which lacked reactivities with MAb 1604 (a MAb specific for a cell surface protein) and MAbs 2600, 1733, 1514, 1412, and 783 (MAbs specific for carbohydrate epitopes on the O antigen of lipopolysaccharide [LPS]). The defect in antibody recognition was shown by genetic crosses and DNA hybridization experiments to be caused by the Tn5 transposon acting as a mutation at a single locus. Quantitative enzyme-linked immunosorbent assays showed that particular mutant strains had no detectable affinity for the specific MAb probe. LPS mutants were resistant to myxophage Mx8, and this provided a selection method for isolating a large number of new LPS mutants. A class of Mx8-resistant mutants lacked reactivity with MAb 1514 and therefore was defective in the O antigen of LPS. A class of Mx1-resistant mutants lacked reactivity with MAb 2254, a MAb specific for a carbohydrate epitope on the core of LPS. A comparison of MAb binding to different mutant strains revealed a principle for mapping epitopes and showed that MAbs 1514 and 2254 recognize side-chain carbohydrates rather than backbone carbohydrates within the LPS molecule.
Full text
PDF


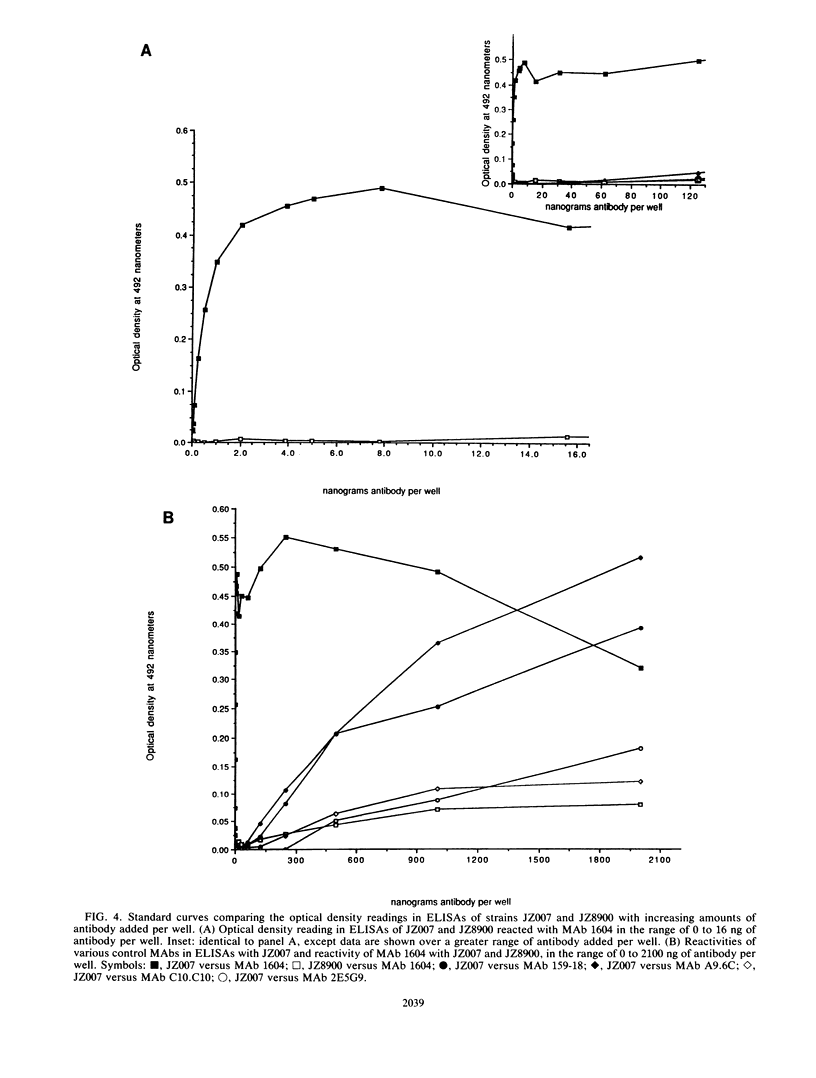


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bretscher A. P., Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978 Feb;133(2):763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. A bacteriophage for Myxococcus xanthus: isolation, characterization and relation of infectivity to host morphogenesis. J Bacteriol. 1966 Mar;91(3):1305–1313. doi: 10.1128/jb.91.3.1305-1313.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Characterization of lipopolysaccharide from Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2028–2032. doi: 10.1128/jb.171.4.2028-2032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Defects in motility and development of Myxococcus xanthus lipopolysaccharide mutants. J Bacteriol. 1989 Apr;171(4):2042–2048. doi: 10.1128/jb.171.4.2042-2048.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Cell surface antigens during submerged development of Myxococcus xanthus examined with monoclonal antibodies. J Bacteriol. 1986 Nov;168(2):505–511. doi: 10.1128/jb.168.2.505-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Jarvis B. W., Dworkin M. Inhibition of development in Myxococcus xanthus by monoclonal antibody 1604. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4505–4508. doi: 10.1073/pnas.84.13.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Stellwag E., Dworkin M. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):11–18. doi: 10.1016/s0769-2609(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Jemmerson R. Multiple overlapping epitopes in the three antigenic regions of horse cytochrome c1. J Immunol. 1987 Jan 1;138(1):213–219. [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Sodergren E., Masuda T., Kaiser D. Systematic isolation of transducing phages for Myxococcus xanthus. Virology. 1978 Jul 1;88(1):44–53. doi: 10.1016/0042-6822(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Orndorff P., Stellwag E., Starich T., Dworkin M., Zissler J. Genetic and physical characterization of lysogeny by bacteriophage MX8 in Myxococcus xanthus. J Bacteriol. 1983 May;154(2):772–779. doi: 10.1128/jb.154.2.772-779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M. Methylation of macromolecules during development in Myxococcus xanthus. J Bacteriol. 1985 Nov;164(2):495–500. doi: 10.1128/jb.164.2.495-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vázquez R., Murillo F. J. Abnormal motility and fruiting behavior of Myxococcus xanthus bacteriophage-resistant strains induced by a clear-plaque mutant of bacteriophage Mx8. J Bacteriol. 1984 Nov;160(2):818–821. doi: 10.1128/jb.160.2.818-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonejans E., Expert D., Toussaint A. Characterization and virulence properties of Erwinia chrysanthemi lipopolysaccharide-defective, phi EC2-resistant mutants. J Bacteriol. 1987 Sep;169(9):4011–4017. doi: 10.1128/jb.169.9.4011-4017.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986 Jun;166(3):837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]