Abstract
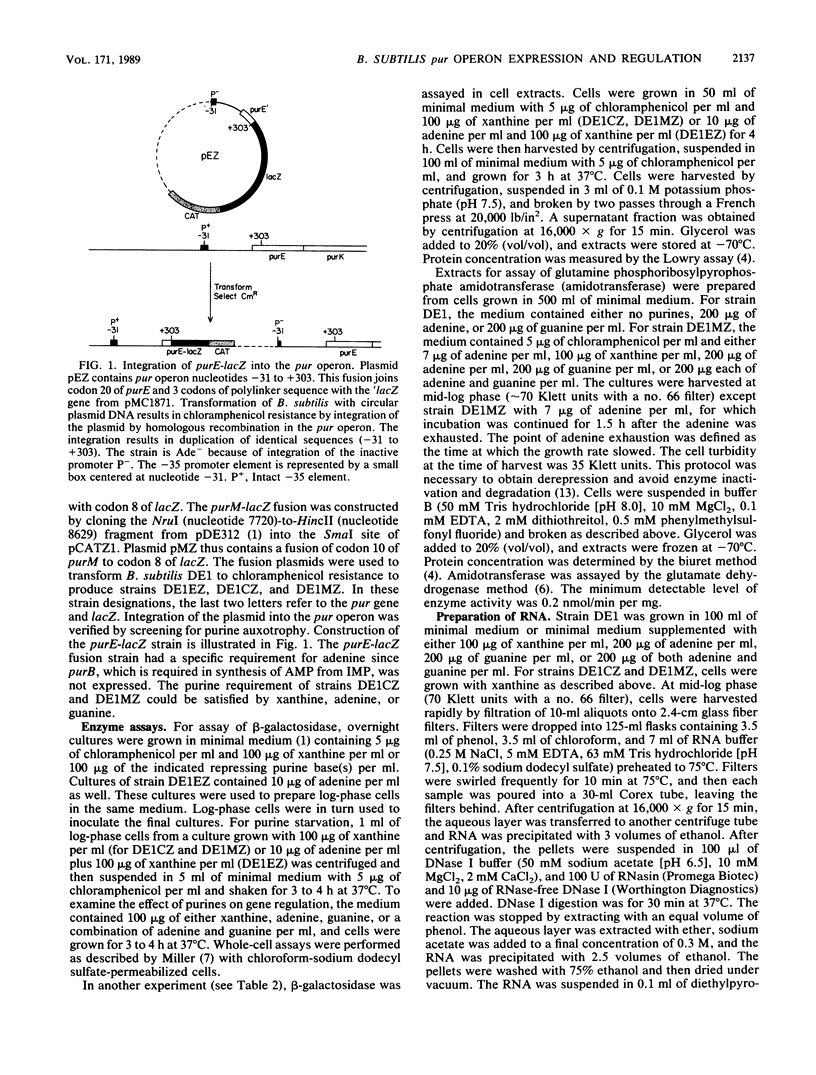
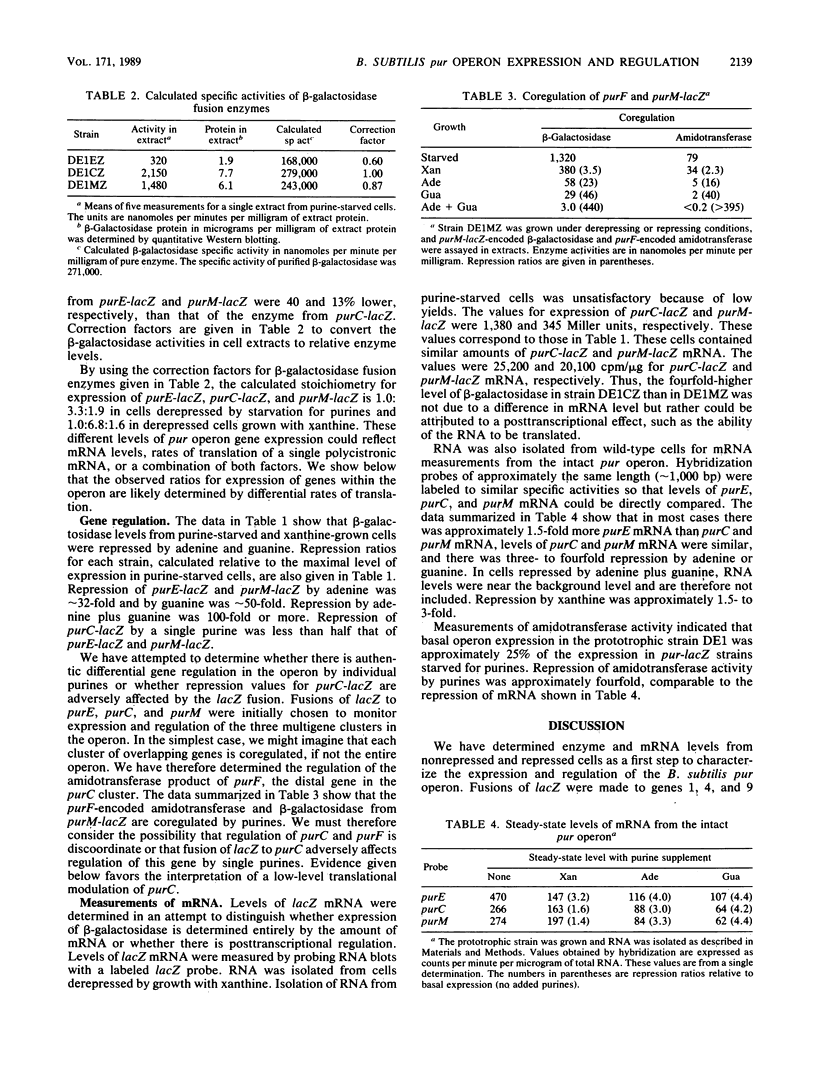
The Bacillus subtilis pur operon is a 12-gene cluster, purEKB-purC(orf)QLF-purMNH(J)-purD, organized in groups of overlapping coding units separated by intercistronic gaps. Translational fusions of Escherichia coli lacZ were constructed to purE, purC, and purM, the first gene of each group. Analyses of gene fusions integrated into the chromosomal pur operon exclude the possibility of internal promoters in intercistronic regions and support the view that transcription is from the single sigma 43 promoter at the 5' end of the operon. Enzyme and mRNA measurements indicate that transcriptional regulation occurs solely at the 5' end of the operon. The relative levels of beta-galactosidase from purE-lacZ, purC-lacZ, and purM-lacZ were determined under repressing and nonrepressing conditions. These results indicate that expression of purC-lacZ was 3.0- to 6.8-fold higher than purE-lacZ because of enhanced translational efficiency. The enhanced translational efficiency of purC-lacZ was accompanied by a partial escape from regulation by purines. This anomalous effect on purC-lacZ was the only suggestion for posttranscriptional regulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebbole D. J., Zalkin H. Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide synthesis. J Biol Chem. 1987 Jun 15;262(17):8274–8287. [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Detection of pur operon-attenuated mRNA and accumulated degradation intermediates in Bacillus subtilis. J Biol Chem. 1988 Aug 5;263(22):10894–10902. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Schairer H. U., Sebald W. Translational initiation frequency of atp genes from Escherichia coli: identification of an intercistronic sequence that enhances translation. EMBO J. 1985 Feb;4(2):519–526. doi: 10.1002/j.1460-2075.1985.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger L. J., Zalkin H. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem. 1979 May 10;254(9):3382–3392. [PubMed] [Google Scholar]
- Nishikawa H., Momose H., Shio I. Regulation of purine nucleotide synthesis in Bacillus subtilis. II. Specificity of purine derivatives for enzyme repression. J Biochem. 1967 Jul;62(1):92–98. doi: 10.1093/oxfordjournals.jbchem.a128640. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxild H. H., Nygaard P. Gene-enzyme relationships of the purine biosynthetic pathway in Bacillus subtilis. Mol Gen Genet. 1988 Jan;211(1):160–167. doi: 10.1007/BF00338408. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in stationary-phase cultures of Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):108–114. doi: 10.1128/jb.121.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]


