Abstract
Tissue-selective androgen receptor modulators (SARMs) demonstrate tissue selectivity in both castrated and intact male rats, behaving as partial agonists in androgenic tissues (i.e. prostate and seminal vesicle), but full agonists in anabolic tissues (i.e. levator ani muscle). The partial agonist activity of SARMs (compounds S-1 and S-4) in the prostate of intact rats suggested that SARM could be used for androgen suppression in the treatment of benign prostate hyperplasia (BPH). This study was designed to explore the mechanisms of action of SARM and to characterize the tissue selectivity of S-1 in intact male rats compared with that of hydroxyflutamide (antiandrogen) and finasteride (5α-reductase inhibitor), two major drugs used for androgen suppression treatment of BPH. In intact male rats, S-1 (5, 10, and 25 mg/kg) selectively decreased the prostate weight with similar efficacy to finasteride (5 mg/kg), without affecting the levator ani muscle or increasing the plasma levels of testosterone, LH, and FSH. Hydroxyflutamide (0.5, 1, 5, 10, and 25 mg/kg), however, decreased both the prostate and levator ani muscle weights without any selectivity and increased plasma hormone levels in a dose-dependent manner. Furthermore, S-1 and S-4 showed very weak inhibitory effects toward transiently expressed type I and II human 5α-reductase (Ki, >20 µM) during in vitro assays. Therefore, although S-1 and finasteride showed very similar suppressive effects in the prostate of intact male rats, they decreased prostate size via different mechanisms of action. S-1 simply worked as androgen receptor partial agonist, whereas finasteride inhibited prostatic 5α-reductase. These studies indicate that SARMs may demonstrate clinical utility as single agent or combination therapy for BPH.
In the past several years, the successful marketing and clinical efficacy of selective estrogen receptor modulators have raised the possibility of developing selective ligands for other members of the nuclear receptor superfamily. The concept of selective androgen receptor (AR) modulators (SARMs) (1, 2), compounds that act as antagonists or weak agonists in the prostate, but act as full agonist in the muscle and pituitary, also emerged. Tissue-selective activation of the AR by SARM could not only greatly improve the side-effect profile of currently available antiandrogens, such as flutamide, that are used in the treatment of BPH, but could also be used to treat muscle-wasting conditions and age-related frailty with less concern for the stimulation of potential prostate diseases.
Nonsteroidal SARMs of several different structural classes have been reported, as recently reviewed by Allan and Sui (3). Our laboratory first discovered a series of flutamide and bicalutamide analogs as a novel class of SARMs in 1998 (4). Additional structural modification improved the binding affinity, intrinsic activity, and pharmacokinetic properties of the compounds (5–8). Both S1 and S4 showed high binding affinity to the AR, with Ki values of 6.1 and 4.0 nm, respectively; these values are similar to that of testosterone (T) and much higher than that of hydroxyflutamide (Ki, 25 nm), but lower than that of dihydrotestosterone (DHT; Ki, 0.2 nm). Our previous in vivo study (9) successfully demonstrated the AR agonist activities of compounds S-1 and S-4 in castrated male rats. In the absence of endogenous androgens, compounds S-1 and S-4 behave as partial agonists in the androgenic tissues (i.e. prostate and seminal vesicle), but act as full agonists in the anabolic tissue (i.e. levator ani muscle) in terms of maintaining these tissue weights after castration. The relative efficacies of S-1 and S-4 in the prostate were 12% and 29%, respectively, compared with that of T propionate (TP). Therefore, in the presence of endogenous androgens, S-1 and S-4 could work as antagonists in the prostate and suppress prostate growth, suggesting their potential application in BPH treatment.
Benign prostatic hyperplasia (BPH) is one of the most common diseases in men older than 50 yr. Urinary obstruction is the main symptom of BPH, and it appears to be caused by both physical obstruction (static or mechanic component) and contractions of smooth muscles under α-receptor-mediated sympathetic stimulation (dynamic component) (10). Currently, both surgical and medical options are available for treatment. Medical therapies include androgen suppression, α-blockade, aromatase inhibitors, and phytotherapy (10–12). α-Blockers improve the symptoms of BPH by reducing the muscular tone, whereas androgen suppression and aromatase inhibition counteract the static component or mechanic enlargement. Androgen suppression primarily causes the regression of the epithelial elements of the prostate whereas aromatase inhibitors are believed to suppress the size of the stromal component and the stromal-epithelial interactions in the prostate.
The most commonly used drugs for androgen suppression include antiandrogens (e.g. hydroxyflutamide) and 5α-reductase inhibitors (e.g. finasteride). Antiandrogens directly block androgen action at the receptor, whereas 5α-reductase inhibitors suppress androgen action by inhibiting the conversion of T to DHT (10, 13). Due to the complete blockage of androgen action in both prostate and pituitary, antiandrogens can cause significant increases in plasma T and LH levels (14). 5α-Reductase inhibitors reduce prostatic and plasma DHT concentrations (15, 16). However, significant increases in prostatic (15) and plasma T (16) levels are commonly observed. The increased prostatic and/or plasma T levels might contribute to increases in prostatic concentrations of estrogen (17), because more substrate is available to aromatase. The increased prostatic estrogen concentration might also promote the proliferation of prostatic tissue, especially the stromal components. In contrast, increased prostatic T can still activate AR, although with lower affinity and intrinsic activity than DHT.
As a partial agonist in the prostate, SARMs may provide a completely new approach for androgen suppression in BPH treatment, with fewer side-effects in the anabolic tissue and pituitary. Therefore, one aim of this study was to characterize the pharmacological effects of S-1 (stronger suppressor in the prostate) in intact male rats in comparison with other androgen suppression treatments: the antiandrogen hydroxyflutamide (active form of flutamide) and the 5α-reductase inhibitor finasteride.
The other aim of the study was to explore the mechanism of action of SARM, specifically investigating the role of 5α-reductase in SARM action in the prostate. Although the partial agonist activity of SARM may fully explain its suppressive effects in the prostate (i.e. replacement of the full agonist T with the partial agonist SARM), possible interactions between SARM and 5α-reductase could also contribute to its tissue selectivity via inhibition of 5α-reductase activity (similar to finasteride) and/or metabolic inactivation of SARM by 5α-reductase.
Materials and Methods
Materials
Compounds S-1 and S-4 (5), hydroxyflutamide, and finasteride were synthesized in our laboratories. The purities of these compounds were greater than 99%, as determined by HPLC. T, TP, polyethylene glycol 300 (PEG300; reagent grade), and dimethylsulfoxide (DMSO; reagent grade) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). The T enzyme immunoassay kit was purchased from Diagnostic Systems Laboratories, Inc. (Webster, TX). Lipofectamine reagent was purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA).
Animals
Male Sprague Dawley rats were purchased from Harlan Biosciences (Indianapolis, IN). The animals were maintained on a 12-h light, 12-h dark cycle with food and water available ad libitum. The animal protocol was reviewed and approved by the institutional laboratory animal care and use committee of Ohio State University.
Experimental design
Intact and castrated male Sprague Dawley rats were used to compare the tissue selectivity of S-1, S-4, and TP. Animals weighing 187–214 g were randomly distributed into eight groups (n = 5/group). On d 0, animals were castrated or received sham operation (intact groups) immediately before sc implantation of a Alzet osmotic pump (model 2002, Durect Corp., Cupertino, CA) prefilled to deliver S-1 (0.5 mg/d), S-4 (0.5 mg/d), or TP (0.5 mg/d). S-1 and S-4 were dissolved in ethanol/PEG300 (5:95, vol/vol). TP was dissolved in ethanol/DMSO/PEG300 (10:35:55, vol/vol/vol). The vehicle for S-1 and S-4 was used in the control groups. Castrated animals were included in this study to provide a direct comparison of the effects of S-1, S-4, and TP in intact and castrated animals during identical treatment conditions (i.e. to avoid variability that might arise during comparison with our previous study).
Additional studies were conducted in intact male rats to compare the tissue selectivity of S-1, hydroxyflutamide, and finasteride. Male Sprague Dawley rats (189–226 g) were randomly distributed into groups of five animals. The intact male rats were treated with hydroxyflutamide (0.5, 1, 5, 10, or 25 mg/kg), finasteride (5 mg/kg), S-1 (0.5, 1, 5, 10, or 25 mg/kg), or vehicle for 3, 6, or 9 d. The drugs were dissolved in DMSO/PEG300 (20:80, vol/vol) and administered via daily sc injections. A group of castrated rats (n = 5) was also included as a control for each time point. Animals were weighed, anesthetized, and killed within 8 h after the last dose. The androgenic and anabolic tissues (ventral prostate, seminal vesicle, and levator ani muscle) were removed and weighed, and blood samples were collected and used for the measurement of serum markers, including FSH, LH, and T. T concentrations were measured using a commercially available enzyme immunoassay kit from Diagnostic Systems Laboratories Inc. FSH and LH levels were measured by the National Hormone and Peptide Program (Dr. A. F. Parlow, University of California, Los Angeles, CA) using rat LH and FSH RIA kits. The organ weights were normalized with the body weights. Percent changes were determined by comparison with intact animals.
5α-Reductase assays
In vitro 5α-reductase assays using cell lysate were performed as described by Thigpen et al. (18). Briefly, COS-1 cells were transiently transfected with cytomegalovirus plasmid for expression of type 1 and type 2 human 5α-reductase (provided by Dr. David W. Russell, Southwestern Medical Center, Dallas, TX) using Lipofectamine (Invitrogen Life Technologies, Inc.). Forty-eight hours after transfection, cells were harvested in PBS, and cell pellets were lysed in 10 mm potassium phosphate (pH 7.0), 150 mm KCl, and 1 mm EDTA with Pro200 homogenizer (Pro Scientific Inc., Oxford, CT). The total protein concentration in the lysate was measured by the Bradford method using BSA as standard. 5α-Reductase assays were conducted in 0.1 m Tris-citrate buffers at the indicated pH (Table 1). Cell lysate (1–3 µg protein) was incubated at 37 C with T and/or S-1 or S-4 for 10–30 min. The reaction was initiated by the addition of NADPH (final concentration, 1mm) and was stopped by adding ice-cold acetonitrile (1:1, vol/vol) containing internal standard for HPLC (Agilent 1100; Agilent Technologies, Inc., Palo Alto, CA) analysis.
TABLE 1.
Inhibition of human 5α-reductase isozymes expressed in transfected COS cells
| Type 1 (pH 7.0) |
Type 2 (pH 5.0) |
|||
|---|---|---|---|---|
| Km (µm) | Vmax [nmol/(min · mg)] | Km (µm) | Vmax [nmol/(min · mg)] | |
| Substrates | ||||
| Testosterone | 4.7 ± 1.5 | 3.0 ± 0.5 | 0.8 ± 0.3 | 2.2 ± 0.5 |
| S-1 | ||||
| S-4 | ||||
| Ki | Ki | |||
| |
||||
| Inhibitors | ||||
| Finasteride (nm) | 484 ± 266 | 23 ± 4 | ||
| S-1 (µm) | 86.5 ± 7.7 | 26.3 ± 5.1 | ||
| S-4 (µm) | 61.2 ± 12.3 | 51.5 ± 19.7 | ||
Vmax, Maximum velocity.
Precipitated protein was pelleted by centrifugation, and the supernatant was subjected to HPLC analysis. T concentrations in the incubate were determined by HPLC using a reverse phase column (3.9 × 300 mm µBondaPak C18, Waters Corp., Milford, MA) and a mobile phase of acetonitrile and deionized water (50:50, vol/vol) at a flow rate of 1 ml/min with a UV absorbance detector set at 254 nm. The intraday coefficient of variation of the HPLC analysis was less than 10% at 0.05 µm. The reaction velocity was calculated based on the decrease in T concentration. Apparent Km, maximum velocity, and 50% inhibitory concentration were determined by nonlinear regression analysis using WinNonlin (version 4.0, Pharsight Corp., Mountain View, CA). Apparent Ki values were derived from the 50% inhibitory concentrations of the inhibition curves.
Statistical analysis
Data are presented as the mean ± sd. Statistical analyses of all parameters were performed by single-factor ANOVA analysis. P < 0.05 was considered statistically significant.
Results
Tissue selectivity of SARM in intact rats
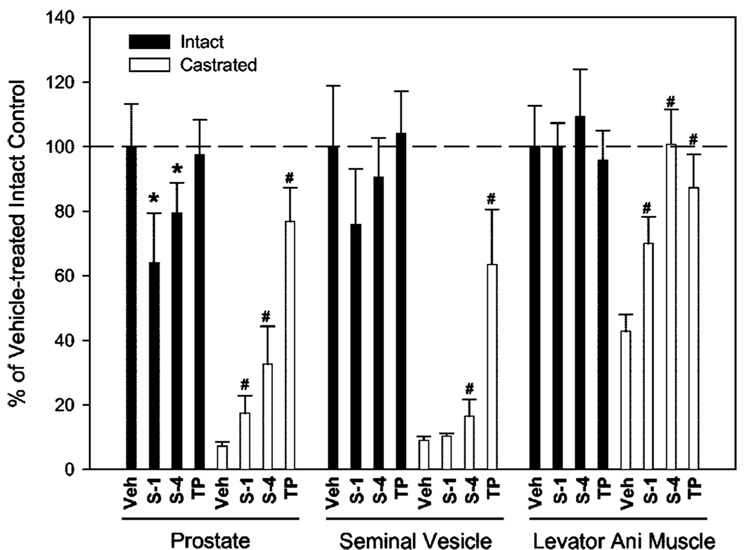
Our previous study (9) characterized the pharmacological activities of S-1 and S-4 in castrated rats. In this study we first characterized the pharmacological effects of S-1 and S-4 in intact rats (Fig. 1). Castrated control animals were included for comparison. A dose rate close to the 50% effective doses of S-1 and S-4 in the androgenic tissues (9) (i.e. 0.5 mg/d) was used for all treatment groups. Identically to our previous study (9), both S-1 and S-4 demonstrated partial agonist activity in the androgenic tissues (prostate) and full agonist activity in the anabolic tissue (levator ani muscle) in castrated animals (Fig. 1, □). S-1 (0.5 mg/d) and S-4 (0.5 mg/d) only partially maintained prostate weight at 17.4% and 32.5%, respectively, and seminal vesicle weight at 10.3% and 16.5%, respectively, of that in vehicle-treated intact animals, whereas TP (0.5 mg/d) increased prostate and seminal vesicle weights to 76.8% and 63.5%, respectively, of that in intact animals. In contrast, treatment of castrated rats with S-1, S-4, and TP increased levator ani muscle weight to 69.9%, 101%, and 87.3%, respectively, of that in intact animals.
FIG. 1.
Tissue selectivity of SARM in intact and castrated male rats (n = 5). Intact and castrated male rats were treated with S-1 (0.5 mg/d), S-4 (0.5 mg/d), and TP (0.5 mg/d) for 14 d. Prostate, seminal vesicle, and levator ani muscle weights were measured at the end of treatment. All organ weights were normalized by body weight and are shown as a percentage of weights in the vehicle-treated intact control group. Data are presented as the mean ± sd. *, P < 0.05 compared with the vehicle-treated intact control group; #, P < 0.05 compared with the vehicle-treated castrated control group.
More importantly, these SARMs demonstrated tissue-selective pharmacological activity in intact animals. Both S-1 and S-4 (0.5 mg/d) significantly decreased prostate weight to 64.1% and 79.4%, respectively, of that in vehicle-treated intact rats, whereas no significant change was seen in the TP-treated group (Fig. 1, ■). Similar trends were observed in seminal vesicle weights (75.9% for S-1 and 90.5% for S-4), although differences were not statistically significant. Furthermore, none of the three treatments caused any significant change in levator ani muscle weight in intact animals.
In summary, S-1 and S-4 demonstrated partial agonist activity in prostate and seminal vesicles of intact and castrated male rats, with 4- to 5-fold higher efficacies and full agonist activity in the levator ani muscle. However, TP demonstrated equal efficacy in prostate, seminal vesicles, and levator ani muscle in both intact and castrated male rats, illustrating its full agonist activity and lack of tissue selectivity. The similar efficacies of TP and the differing efficacies of S-1 and S-4 in androgenic and anabolic tissues provided direct evidence of the tissue-selective pharmacological properties of SARM in both intact and castrated male animals.
Comparison of the pharmacological effects of S-1, hydroxyflutamide, and finasteride in intact rats
The tissue-selective and inhibitory effects of S-1 in the prostate of intact animals suggested that this new class of SARM might be useful for the treatment of BPH. Therefore, the pharmacological effects of SARM in intact animals were characterized in more detailed dose-response and time-course studies and were compared with the effects of other androgen-suppressive agents used for BPH treatment, hydroxyflutamide (antiandrogen) and finasteride (5α-reductase inhibitor). S-1 tended to show larger inhibitory effects in the prostate of intact animals (Fig. 1) and was chosen for this experiment.
Because both S-1 and hydroxyflutamide share the same target (i.e. AR), multiple doses of S-1 and hydroxyflutamide were used to characterize the dose-response relationship. Furthermore, multiple time points were included to compare the onset of the effects at different doses. Finasteride was given at 5 mg/kg·d, the lowest dose that produces the maximum inhibitory effects in the prostate (19).
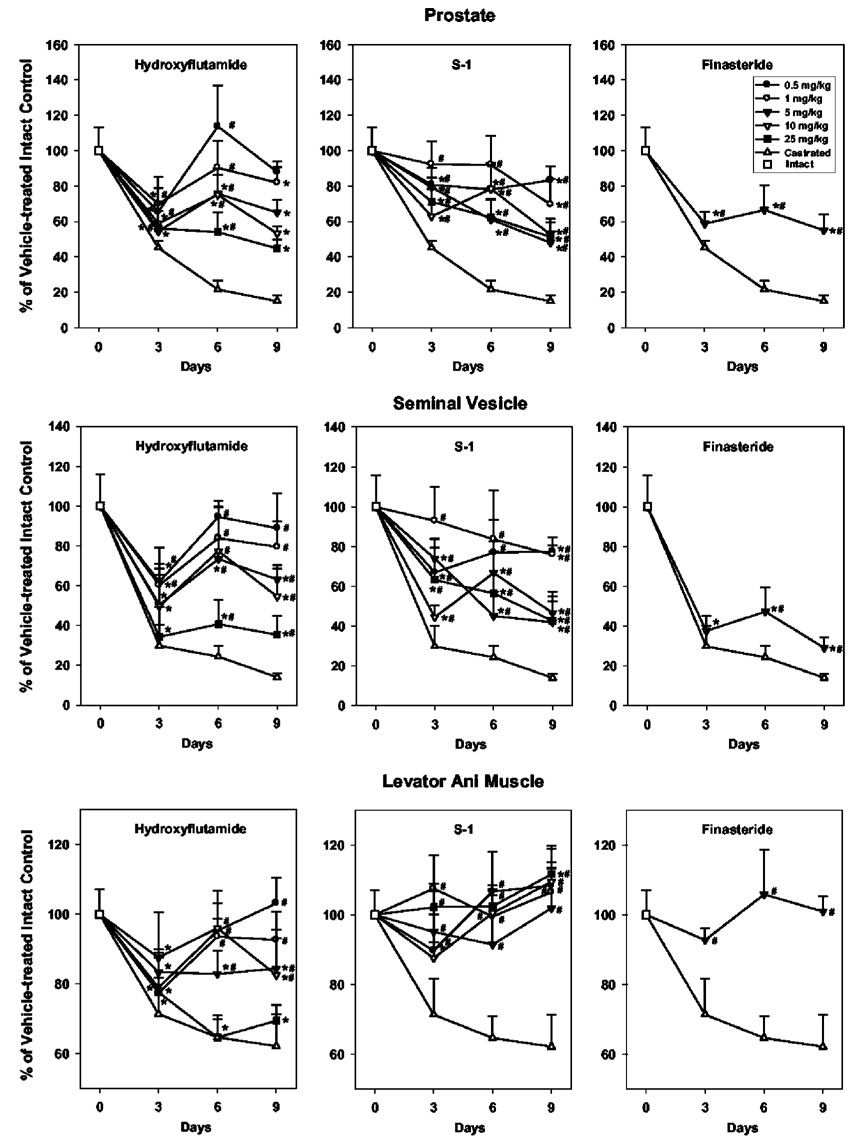
Castration caused rapid decreases in both androgenic and anabolic tissues. After 3, 6, and 9 d of treatment, prostate weight decreased to 45%, 22%, and 15%, respectively, of that in intact animals; seminal vesicle weight decreased to 30%, 24%, and 14%, respectively, of that in intact animals; and levator ani muscle weight decreased to 71%, 65%, and 62%, respectively, of that in intact animals (Fig. 2).
FIG. 2.
Comparison of the tissue selectivity of hydroxyflutamide, S-1, and finasteride in ventral prostate, seminal vesicle, and levator ani muscle in intact male rats after different treatment periods (n = 5). Intact male rats were treated with hydroxyflutamide (0.5, 1, 5, 10, or 25 mg/kg), S-1 (0.5, 1, 5, 10, or 25 mg/kg), and finasteride (5 mg/kg) for 3, 6, and 9 d. Vehicle-treated intact and castrated groups were also included as controls. Prostate, seminal vesicle, and levator ani muscle weights were measured at the end of each treatment period. All organ weights were normalized by body weight and are shown as a percentage of weights in the vehicle-treated intact control group. Data are presented as the mean ± sd. *, P < 0.05 compared with the vehicle-treated intact control group at the same time point; #, P < 0.05 compared with the vehicle-treated castrated control group at the same time point.
Antiandrogens block the binding of endogenous androgens, including T and DHT, to the AR. Hydroxyflutamide treatment caused very similar effects as castration in both androgenic (DHT-dependent) and anabolic (DHT-independent) tissues. After only 3 d of treatment at all doses tested, hydroxyflutamide significantly decreased the tissue weights of prostate, seminal vesicle, and levator ani muscle to near the castration level measured at the same time point. However, fluctuations in the changes were observed over time, and the dose-response relationship was only observed after 9 d of treatment. At 25 mg/kg, hydroxyflutamide significantly decreased prostate, seminal vesicle, and levator ani muscle weights to 45%, 35%, and 69%, respectively, of intact levels. Although hydroxyflutamide (5 mg/kg) decreased prostate weight to a similar extent (65%) as finasteride (55%) at the same dose after 9 d of treatment, it also significantly decreased levator ani muscle weight to 84%, showing its lack of tissue selectivity.
Due to the tissue-specific expression of 5α-reductase, finasteride only caused significant decreases in DHT-dependent tissues (prostate and seminal vesicle) without affecting DHT-independent tissue (levator ani muscle). At 5 mg/kg, finasteride caused significant decreases in prostate and seminal vesicle weights in as little as 3 d, and the changes in tissue weights were similar after 3, 6, and 9 d of treatment. Prostate and seminal vesicle weights decreased to 55% and 29%, respectively, of the intact level after 9 d of treatment, whereas no significant change in levator ani muscle weight was observed.
Intact male rats were also treated with S-1 at the same doses as hydroxyflutamide for 3, 6, and 9 d. After 9 d of treatment, S-1 selectively decreased prostate and seminal vesicle weights without affecting the levator ani muscle in a dose-dependent manner. The maximum inhibitory effects were observed at the higher doses (i.e. ≥5 mg/kg). Prostate and seminal vesicle weights were decreased to a similar extent (50% and 45%, respectively, of the intact level), comparable to the efficacy of finasteride (55% and 29%, respectively; Fig. 3). Although S-1 showed similar tissue selectivity as finasteride, fluctuations in tissue responses were observed during 3 and 6 d of treatment. Additional doses of finasteride were not examined. However, fluctuations in the tissue responses on d 3, 6, and 9 were also observed for this drug (Fig. 2). As a whole, finasteride and S-1 showed better tissue selectivity than hydroxyflutamide for DHT-dependent tissues (prostate and seminal vesicle), which would cause less adverse effects in other peripheral tissues during treatment of BPH.
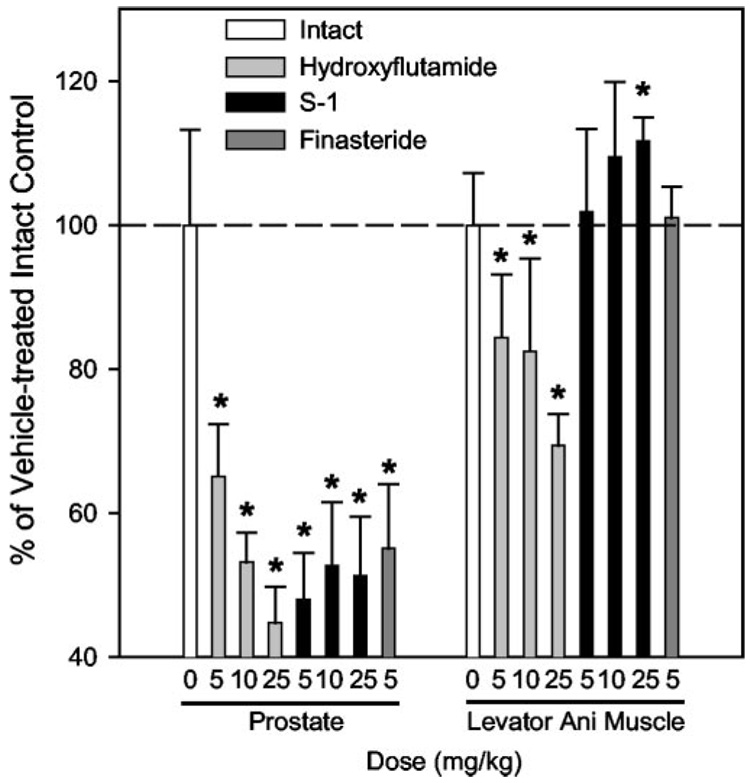
FIG. 3.
Comparison of the anabolic effects of hydroxyflutamide, S-1, and finasteride at doses that showed equal potency in the prostate after 9 d of treatment (n = 5). After 9 d of treatment, hydroxyflutamide (5, 10, or 25 mg/kg), S-1 (5, 10, or 25 mg/kg), and finasteride (5 mg/kg) decreased prostate weight in intact rats to a similar extent. The anabolic effects of these treatments in levator ani muscle were also compared. Data are presented as the mean ± sd.*, P < 0.05 compared with the vehicle-treated intact control group.
As mentioned above, the tissue weights in all drug-treated animals fluctuated over time. Stable dose-response relationships were only observed in the hydroxyflutamide and S-1 groups after 9 d of treatment. The fluctuations in tissue responses to these treatments could have been due to fluctuations in plasma concentrations of T. Thus, we also measured plasma concentrations of T, LH, and FSH to compare the endocrine properties of these compounds.
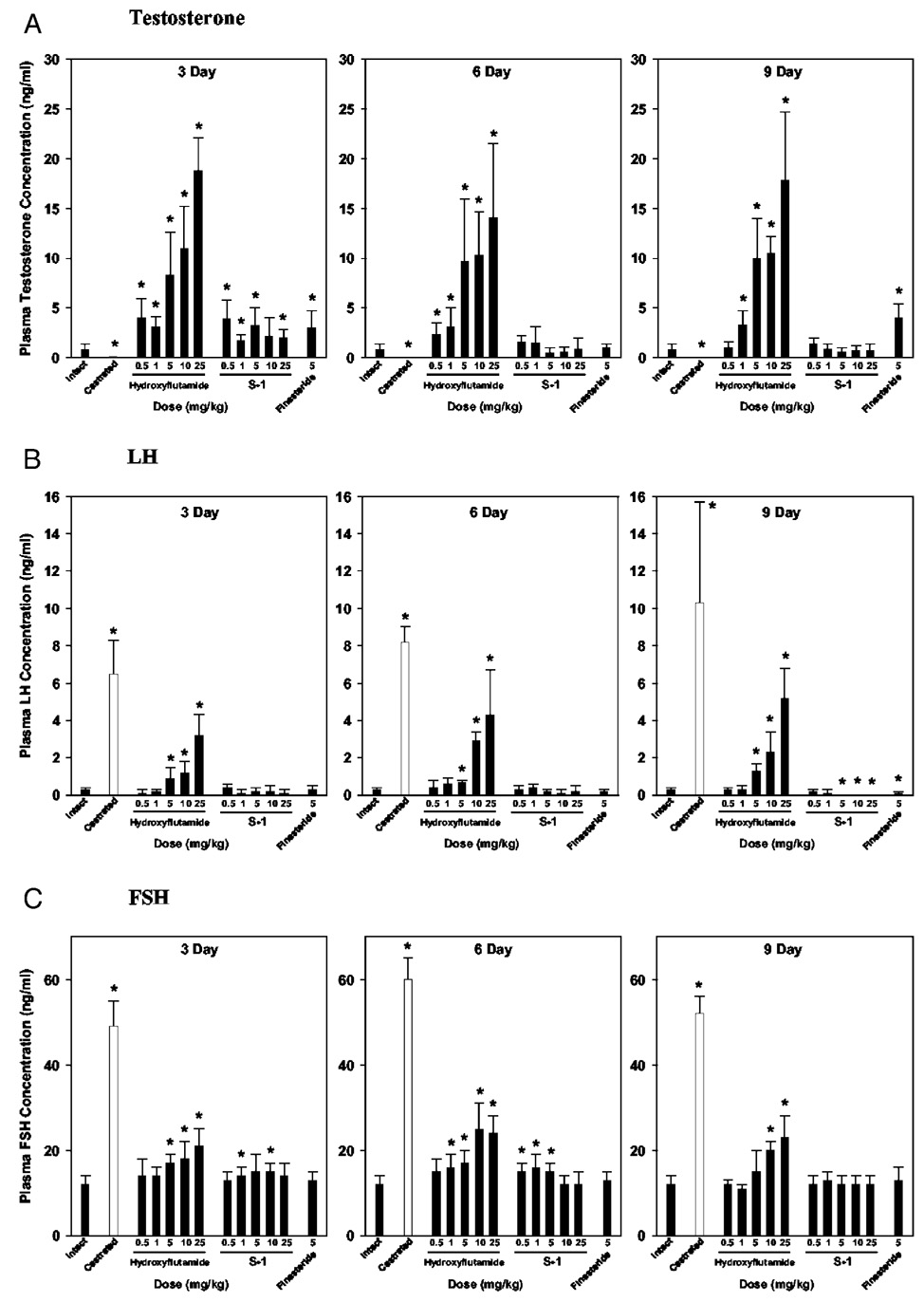
Hydroxyflutamide treatment significantly increased plasma T, LH, and FSH concentrations in intact animals in a dose-dependent manner (Fig. 4), largely by blocking the negative feedback regulation of T in pituitary and hypothalamus. The initial blockage of the feedback loop caused rapid increases in plasma T, LH, and FSH levels and probably contributed to the transient increase in tissue weights at 6 d. However, once a new balance between androgen blockage and feedback increase in plasma hormone concentration was established (i.e. after 9 d of treatment), dose-response relationships were more clearly defined.
FIG. 4.
Effects of hydroxyflutamide, S-1, and finasteride on plasma concentrations of T, LH, and FSH in intact male rats after different treatment periods (n = 5). Intact male rats were treated with hydroxyflutamide (0.5, 1, 5, 10, or 25 mg/kg), S-1 (0.5, 1, 5, 10, or 25 mg/kg), and finasteride (5 mg/kg) for 3, 6, and 9 d. Vehicle-treated intact and castrated groups were also included as controls. Plasma concentrations of T, LH, and FSH were measured at the end of each treatment period. Data are presented as the mean ± sd. *, P < 0.05 compared with the intact control group at the same time point.
Similar to hydroxyflutamide, S-1 caused transient increases in plasma T (Fig. 4A) after 3 d of treatment, which could be due to its competitive binding to AR. The plasma T level returned to normal after 6 and 9 d of treatment. Different from hydroxyflutamide, S-1 is a partial AR agonist. Thus, it did not block the negative feedback loop, and plasma LH and FSH levels (Fig. 4, B and C) were less affected by S-1 treatment. Plasma FSH levels were slightly increased after 3 and 6 d of treatment, but returned to normal after 9 d of treatment. No significant changes in plasma LH levels were observed after 3 and 6 d of treatment with S-1. However, longer (i.e. 9 d) treatment and higher doses (i.e. 5, 10, and 25 mg/kg) of S-1 significantly suppressed LH secretion to undetectable levels, similar to the effects seen in intact rats treated with exogenous T (20).
Finasteride, similar to S-1, did not cause significant increases in plasma FSH concentrations in intact male rats. However, finasteride significantly increased plasma T levels due to the inhibition of 5α-reductase at tissue level and thus decreased plasma LH levels in intact animals after 9 d of treatment.
As demonstrated in Fig. 2, all three drugs were able to decrease prostate size in intact animals. Hydroxyflutamide (5, 10, and 20 mg/kg), S-1 (5, 10, and 20 mg/kg), and finasteride (5 mg/kg) were similarly potent in decreasing prostate weight in intact animals (Fig. 3). However, compared with equipotent doses of hydroxyflutamide and finasteride, S-1 showed a better pharmacological profile in other tissues. 1) S-1 did not cause significant decreases in levator ani muscle. In fact, the 25 mg/kg dose significantly increased the muscle weight by 10%, demonstrative of the strong agonist activity of S-1 in muscle. 2) S-1 did not increase plasma T, LH, and FSH levels in intact animals, showing its agonist activity in the pituitary, whereas hydroxyflutamide dramatically increased T, LH, and FSH levels, and finasteride significantly increased plasma T. In summary, S-1 showed suppressive effects in the prostates of intact male rats, with similar potency as hydroxyflutamide and finasteride, two clinically approved reagents for the treatment of BPH, suggesting that SARM might represent a new approach to treat BPH.
SARM and 5α-reductase
As discussed above, S-1 and finasteride behaved similarly in androgen-responsive tissues and showed very similar effects on endocrine physiology. The tissue selectivity of finasteride is thought to arise from the tissue-specific expression of 5α-reductase. Because S-1 is an AR agonist and mimics many of the pharmacological properties of T, we speculated that interactions with 5α-reductase might also contribute to its tissue-selective pharmacological effects.
In vitro enzyme assays with transiently expressed human type 1 or 2 5α-reductase showed that S-1 and S-4 are not substrates for either type of 5α-reductase. Experiments were performed at the optimum pH, as determined by Thigpen et al. (18): type 1 isozyme at pH 7.0 and type 2 isozyme at pH 5.0. Inhibition studies showed that S-1 inhibited 5α-reductase activity at high micromolar concentrations (Table 1), with Ki values of 86.5 and 26.3 µm for type 1 and type 2 5α-reductase, respectively, whereas finasteride showed more potent activity, with Ki values of 484 and 23 nm, respectively, which were close to the previously reported values (i.e. 325 and 12 nm) (18).
Discussion
Endogenous T is converted by 5α-reductase to a more potent form, DHT, in many tissues, including the prostate. Two 5α-reductase isozymes have been identified in human and rats (21–23): type 1 and type 2 5α-reductase, with prostate expressing predominantly the type 2 isozyme, and liver and skin primarily expressing the type 1 isozyme. Both isozymes are expressed at a much lower level in other peripheral tissues, including skeletal muscle and pituitary (22,23). Due to the tissue-specific expression of 5α-reductase, prostatic DHT concentrations are much higher than prostatic T concentrations (15, 24), and DHT is believed to be the major endogenous androgen responsible for prostate growth and maintenance. Therefore, prostate is commonly considered a DHT-dependent tissue, whereas pituitary and skeletal muscle are considered DHT-independent tissues.
AR and 5α-reductase are the major targets for androgen suppression during treatment of BPH. SARMs and antiandrogens are AR ligands and suppress DHT action by competitively binding to the AR, whereas 5α-reductase inhibitors suppress DHT action by decreasing its formation in the prostate.
Antiandrogens (i.e. flutamide and bicalutamide) are AR antagonists. These drugs block AR binding and suppress tissue uptake of circulating T (25), causing tissue androgen ablation similar to that after castration. Although hydroxyflutamide efficiently decreases prostate volume in male rats (26, 27) and in BPH patients (28, 29), the action is not tissue selective, and significant androgen suppression in other tissues causes major side-effects, including 1) symptoms of androgen depletion: hot flashes, impotence (29), and suppression of the anabolic effects of androgen in the muscle; and 2) breast tenderness (42–52%) and gynecomastia (12–17%) (29) caused by increased estrogen concentration in tissues as a result of increased circulating T levels after the blockage of the negative feedback loop at the pituitary and hypothalamus levels. Therefore, an AR ligand with better tissue selectivity that acts as an antagonist or a weak agonist in the prostate, but acts as an agonist in the pituitary, is needed to reduce the adverse effects of antiandrogens. The discovery of the nonsteroidal SARMs provides a means by which this goal may be achieved.
S-1 showed very low androgenic activity in the prostate (<15% the efficacy of T), which makes it a strong antagonist in DHT-dependent tissues. In intact male rats, S-1 decreased prostate size by half, showing a similar potency as hydroxyflutamide. However, in DHT-independent tissues, S-1 worked as a full AR agonist, demonstrating greatly improved tissue selectivity. Its agonist activity in pituitary maintained the feedback regulation of endogenous T and LH secretion. Thus, S-1 did not increase plasma T and LH levels and would probably be devoid of estrogenic side-effects commonly encountered with antiandrogens. Its agonist activity in other peripheral tissues will help reduce androgen depletion syndromes. Its strong agonist activity in the skeletal muscle could also be used to treat muscle wasting and age-related frailty.
Also, prostate weight in S-1-treated animals was significantly correlated with serum T concentrations in the 9-d treatment group (data not shown). However, it is important to note that serum T concentrations were unchanged, and serum LH concentrations were reduced by S-1 treatment. Preclinical studies of bicalutamide in rats suggested that it was a peripherally selective antiandrogen (27). However, human clinical trials showed that bicalutamide influences serum LH and T levels. The ability of S-1 to influence serum LH levels in rats combined with the observed correlation between serum T and prostate weight in animals treated with S-1 for 9 d suggest that the pharmacological effects of S-1 on the prostate were at least partially due to changes in serum T concentrations.
Different from SARM and antiandrogen, finasteride decreases circulating (16) and prostatic (15) DHT concentrations by inhibiting type 2 5α-reductase. Compared with antiandrogen, finasteride showed greatly improved tissue selectivity and endocrine properties due to the tissue-specific expression of type 2 5α-reductase (22, 23). No significant increases in circulating LH and FSH levels were observed in either animal studies or BPH patients (16). However, even without changing feedback regulation of endogenous T, effective inhibition of the 5α-reductase in tissues still causes the elevation of both circulating and prostatic T levels in rats (19, 24, 30, 31) and humans (16, 32). Side-effects, including breast tenderness and gynecomastia, are observed in some BPH patients (<1%) (33–35), but are significantly improved compared with the side-effects of flutamide (15–52%) (29). Because it decreases DHT formation in the prostate, finasteride also increases the intraprostatic T level in a reciprocal fashion (15). T by itself can also stimulate prostate epithelial elements, although with lower potency than that of DHT (36,37). Because more T is now available for aromatase, the intraprostatic estrogen to androgen ratio was significantly increased by finasteride (17) as well, which could attenuate its overall suppressive effects on the prostate by further stimulating the stromal component. Although combination therapy using 5α-reductase inhibitor and aromatase inhibitor has been proposed as a more potent method to suppress both the epithelial and stromal components, the effects have not been confirmed by large scale placebo-controlled clinical trials.
As shown in Table 1, S-1 has very low affinity for 5α-reductase. Our pharmacokinetics and tissue distribution studies of S-1 and/or S-4 have shown that neither plasma nor tissue concentrations of S-1 at similar doses were high enough for effective inhibition of the prostatic 5α-reductase. Therefore, effective inhibition of the 5α-reductase could not be achieved in vivo. Although, finasteride and S-1 shared similar potency and tissue selectivity in suppressing prostate growth in intact male rats, and both are somehow related to the DHT dependency of the prostate, they work on different targets and have completely different mechanisms of action. Compared with finasteride, S-1 showed potential advantage for BPH treatment by effectively maintaining the balance of the feedback regulation of endogenous T, as reflected by the unchanged circulating T level after 9 d of treatment. The plasma LH concentration was even decreased after 9 d of treatment with S-1, suggesting that an additional decrease in circulating T could occur after longer treatment. Also, the lack of interaction between SARM and estrogen receptors, SARM, and aromatase (data not shown) precludes possible estrogenic effects of S-1 itself. Furthermore, S-1 will not increase prostatic estrogen levels by inhibiting 5α-reductase, so stimulation of the stromal components will not be of concern during SARM treatment.
Other side-effects of finasteride include decreased libido, decreased ejaculation, and impotence (33–35) and occur in less than 10% of patients. The effects of SARMs on libido, sexual behavior, and spermatogenesis are under investigation in our laboratory and will be the subject of forthcoming reports.
In summary, SARM (S-1) suppressed prostate growth in intact animals with similar potency and efficacy as hydroxyflutamide and finasteride, but with greatly improved tissue selectivity, endocrine properties, and oral bioavailability (38). The tissue selectivity of SARM is not due to 5α-reductase inhibition, but, instead, is caused by its partial agonist activity in the prostate. The full agonist activity of SARM in other tissues, such as muscle and pituitary, greatly improved the safety of the treatment. Therefore, SARM represents a novel approach, with high tissue selectivity, for BPH treatment.
Acknowledgments
This work was supported by grants from the NIDDK (R01-DK-59800-01) and GTx, Inc. (to J.T.D. and D.D.M.).
Abbreviations
- AR
Androgen receptor
- BPH
benign prostate hyperplasia
- DHT
dihydrotestosterone
- DMSO
dimethylsulfoxide
- PEG
polyethylene glycol
- SARM
selective androgen receptor modulator
- T
testosterone
- TP
testosterone propionate
References
- 1.Zhi L, Martinborough E. Selective androgen receptor modulators (SARMs) Annu Rep Med Chem. 2001;36:169–180. [Google Scholar]
- 2.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84:3459–3462. doi: 10.1210/jcem.84.10.6122. [DOI] [PubMed] [Google Scholar]
- 3.Allan GF, Sui Z. Therapeutic androgen receptor ligands. NURSA e-Journal. 2003;1 doi: 10.1621/nrs.01009. ID 3.09172003.09172001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Discovery of nonsteroidal androgens. Biochem Biophys Res Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 5.Marhefka CA, Gao W, Chung K, Kim J, He Y, Yin D, Bohl C, Dalton JT, Miller DD. Design, synthesis, and biological characterization of metabolically stable selective androgen receptor modulators. J Med Chem. 2004;47:993–998. doi: 10.1021/jm030336u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Yin D, Perera M, Kirkovsky L, Stourman N, Li W, Dalton JT, Miller DD. Noval nonsteroidal ligands with high binding affinity and potent functional activity for the androgen receptor. Eur J Med Chem. 2002;37:619–634. doi: 10.1016/s0223-5234(02)01335-1. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]
- 8.Yin D, Xu H, He Y, Kirkovsky LI, Miller DD, Dalton JT. Pharmacology, pharmacokinetics, and metabolism of acetothiolutamide, a novel nonsteroidal agonist for the androgen receptor. J Pharmacol Exp Ther. 2003;304:1323–1333. doi: 10.1124/jpet.102.040832. [DOI] [PubMed] [Google Scholar]
- 9.Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT. Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther. 2003;304:1334–1340. doi: 10.1124/jpet.102.040840. [DOI] [PubMed] [Google Scholar]
- 10.Chacon A, Monga M. Medical management of benign prostatic hyperplasia. Geriatr Nephrol Urol. 1999;9:39–48. doi: 10.1023/a:1008308819463. [DOI] [PubMed] [Google Scholar]
- 11.Monga M. Update on the medical management of benign prostatic hyperplasia. Int Urol Nephrol. 2002;33:67–68. doi: 10.1023/a:1014405019814. [DOI] [PubMed] [Google Scholar]
- 12.Schroder FH. Changing approches in the treatment of benign prostatic hyperplasia. Eur Urol. 1991;20 Suppl 1:63–67. doi: 10.1159/000471749. [DOI] [PubMed] [Google Scholar]
- 13.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- 14.Geller J. Benign prostatic hyperplasia: pathogenesis and medical therapy. J Am Geriatr Soc. 1991;39:1208–1216. doi: 10.1111/j.1532-5415.1991.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 15.McConnell JD, Wilson JD, George FW, Geller J, Pappas F, Stoner E. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1992;74:505–508. doi: 10.1210/jcem.74.3.1371291. [DOI] [PubMed] [Google Scholar]
- 16.Gormley GJ, Stoner E, Rittmaster RS, Gregg H, Thompson DL, Lasseter KC, Vlasses PH, Stein EA. Effects of finasteride (MK-906), a 5α-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990;70:1136–1141. doi: 10.1210/jcem-70-4-1136. [DOI] [PubMed] [Google Scholar]
- 17.Shibata Y, Fukabori Y, Ito K, Suzuki K, Yamanaka H. Comparison of histological compositions and apoptosis in canine spontaneous benign prostatic hyperplasia treated with androgen suppressive agents chlormadinone acetate and finasteride. J Urol. 2001;165:289–293. doi: 10.1097/00005392-200101000-00081. [DOI] [PubMed] [Google Scholar]
- 18.Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J Biol Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- 19.Shoa TC, Kong A, Marafelia P, Cunningham GR. Effects of finasteride on the rat ventral prostate. J Androl. 1993;14:79–86. [PubMed] [Google Scholar]
- 20.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 21.Russell DW, Berman DM, Bryant JT, Cala KM, Davis DL, Landrum CP, Prihoda JS, Silver RI, Thigpen AE, Wigley WC. The molecular genetics of steroid 5α-reductases. Rec Prog Horm Res. 1994;49:275–284. doi: 10.1016/b978-0-12-571149-4.50018-0. [DOI] [PubMed] [Google Scholar]
- 22.Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J Clin Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- 24.Rittmaster RS, Manning AP, Wright AS, Thomas LN, Whitefield S, Norman RW, Lazier CB, Rowden G. Evidence for atrophy and apoptosis in the ventral prostate of rats given the 5α-reductase inhibitor finasteride. Endocrinology. 1995;136:741–748. doi: 10.1210/endo.136.2.7835306. [DOI] [PubMed] [Google Scholar]
- 25.Kondo Y, Homma Y, Aso Y, Kawabe K, Mieda M, Takahashi H. Relative potency of antiandrogens with reference to intracellular testosterone in the rat prostate. Prostate. 1996;29:146–152. doi: 10.1002/(SICI)1097-0045(199609)29:3<146::AID-PROS1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Folkerts A, Stahl B, Kayser M. Feasibility and potential gains of enhancing the subacute rat study protocol (OECD test guideline no. 407) by additional parameters selected to determine endocrine modulation. A pre-validation study to determine endocrine-mediated effects of the antiandrogenic drug flutamide. Arch Toxicol. 2001;75:65–73. doi: 10.1007/s002040100214. [DOI] [PubMed] [Google Scholar]
- 27.Furr BJ, Tucker H. The preclinical development of bicalutamide: pharmacodynamics and mechanism of action. Urology. 1996;47:13–25. 29–32. doi: 10.1016/s0090-4295(96)80003-3. [DOI] [PubMed] [Google Scholar]
- 28.Kolvenbag GJ, Blackledge GR, Gotting-Smith K. Bicalutamide (Casodex) in the treatment of prostate cancer: history of clinical development. Prostate. 1998;34:61–72. doi: 10.1002/(sici)1097-0045(19980101)34:1<61::aid-pros8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Narayan P, Trachtenberg J, Lepor H, Debruyne FM, Tewari A, Stone N, Das S, Jimenez-Cruz JF, Shearer R, Klimberg I, Schellhammer PF, Costello AJ. A dose-response study of the effect of flutamide on benign prostatic hyperplasia: results of a multicenter study. Urology. 1996;47:497–504. doi: 10.1016/s0090-4295(99)80484-1. [DOI] [PubMed] [Google Scholar]
- 30.Prahalada S, Rhodes L, Grossman SJ, Heggan D, Keenan KP, Cukierski MA, Hoe CM, Berman C, van Zwieten MJ. Morphological and hormonal changes in the ventral and dorsolateral prostatic lobes of rats treated with finasteride, a 5α-reductase inhibitor. Prostate. 1998;35:157–164. doi: 10.1002/(sici)1097-0045(19980515)35:3<157::aid-pros1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.George FW. Androgen metabolism in the prostate of the finasteride-treated, adult rat: a possible explanation for the differential action of testosterone and 5α-dihydrotestosterone during development of the male urogenital tract. Endocrinology. 1997;138:871–877. doi: 10.1210/endo.138.3.5009. [DOI] [PubMed] [Google Scholar]
- 32.Stoner E. 5α-Reductase inhibitors for the treatment of benign prostatic hyperplasia. Rec Prog Horm Res. 1994;49:285–292. doi: 10.1016/b978-0-12-571149-4.50019-2. [DOI] [PubMed] [Google Scholar]
- 33.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG, Nickel JC, Wang DZ, Taylor AM, Waldstreicher J. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 34.Lowe FC, McConnell JD, Hudson PB, Romas NA, Boake R, Lieber M, Elhilali M, Geller J, Imperto-McGinely J, Andriole GL, Bruskewitz RC, Walsh PC, Bartsch G, Nacey JN, Shah S, Pappas F, Ko A, Cook T, Stoner E, Waldstreicher J Finasteride Study Group. Long-term 6-year experience with finasteride in patients with benign prostatic hyperplasia. Urology. 2003;61:791–796. doi: 10.1016/s0090-4295(02)02548-7. [DOI] [PubMed] [Google Scholar]
- 35.Marberger MJ. Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. PROWESS Study Group. Urology. 1998;51:677–686. doi: 10.1016/s0090-4295(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 36.Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest. 1996;98:2558–2563. doi: 10.1172/JCI119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5α-reductase. Endocrinology. 1999;140:4509–4515. doi: 10.1210/endo.140.10.7039. [DOI] [PubMed] [Google Scholar]
- 38.Kearbey JD, Wu D, Gao W, Miller DD, Dalton JD. Pharmacokinetics of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide, a nonsteroidal selective androgen receptor modulator. Xenobiotica. 2004;34:273–280. doi: 10.1080/0049825041008962. [DOI] [PMC free article] [PubMed] [Google Scholar]