Abstract
Exposure of fishes to environmental estrogens is known to affect sexual development and spawning, but little information exists regarding effects on gametes. This study evaluated embryonic survival of offspring from male rainbow trout (Oncorhynchus mykiss) exposed to 17α-ethynylestradiol (EE2) using an in vitro fertilization protocol. Males were exposed at either 1800 or 6700 degree days (°d) (i.e. 161 or 587 days post-fertilization (dpf)) to test for effects on testes linked to reproductive ontogeny. At 1800°d, fish were beginning testicular differentiation and were exposed to 109 ng EE2/l for 21 days. At 6700°d, fish have testes containing spermatocytes and spermatids and were exposed for 56 days to either 0.8, 8.3, or 65 ng EE2/l. Semen was collected at full sexual maturity in each group and used to fertilize eggs pooled from several non-exposed females. Significant decreases in embryonic survival were observed only with the 6700°d exposure. In 0.8 and 8.3 ng EE2/l treatments, embryo survival was significantly reduced at 19 dpf when compared with the control. In contrast, an immediate decrease in embryonic survival at 0.5 dpf was observed in the 65 ng EE2/l treatment. Blood samples collected at spawning from 6700°d exposed males revealed a significant decrease in 11-ketotestosterone and a significant increase in luteinizing hormone levels for the 65 ng EE2/l treatment when compared with the other treatment groups. Results indicate that sexually maturing male rainbow trout are susceptible to EE2 exposure with these fish exhibiting two possible mechanisms of reduced embryonic survival through sperm varying dependant on EE2 exposure concentrations experienced.
Introduction
Increasingly, detrimental reproductive effects in aquatic organisms due to environmental estrogens have been reported in both marine and freshwater ecosystems. These contaminants are widely distributed in aquatic environments and are identified through both direct chemical detection in surface waters as well as their known biological impacts in North America (Kolpin et al. 2002, Goksoyr 2006, Woodling et al. 2006), South America (Orrego et al. 2006), Europe (Jobling et al. 1998, Liney et al. 2005), and Asia (Hashimoto et al. 2000). The effects of environmental estrogens are primarily mediated by either their binding to or interference with estrogen receptors. This interaction results in direct or indirect obstruction of the endocrine system, thus inhibiting reproduction. Notable environmental estrogens include the synthetic human birth control pharmaceutical 17α-ethynylestradiol (EE2), the plasticizing agent bisphenol A, pesticides such as dichloro-diphenyl-trichloroethane (DDT), and the detergent metabolite nonylphenol, among others. Animals exposed to environmental estrogens have exhibited defects in sexual development, sexual behavior, and reproduction organ malformation (Blomqvist et al. 2006, Furuya et al. 2006, Ma & Sassoon 2006). Besides these effects, fishes display abnormal intersexuality, reduced spawning success, reduced female fecundity, delayed male gonadal differentiation, reproductive duct malformation, and complete sex reversal (Jobling et al. 1998, 2003, Scholz & Gutzeit 2000, Van den Belt et al. 2001, 2003, Balch et al. 2004, Nash et al. 2004, Versonnen & Janssen 2004, Mills & Chichester 2005, Campbell et al. 2006).
Environmental estrogens in aquatic environments come from both point and non-point sources, including agricultural runoff and pharmaceutical products passing through sewage treatment facilities (Ingerslev et al. 2003, Tashiro et al. 2003, Wang et al. 2004, Matthiessen et al. 2006). Sewage treatment plant effluent, the most frequent source of environmental estrogen contamination, can be directly released into adjacent surface waters. Documented reports of fishes in these waters with both physiological and developmental problems have been observed (Hashimoto et al. 2000, Jobling et al. 2003, Liney et al. 2005, Rempel et al. 2006). Sewage effluents may contain many environmental estrogens, but of particular concern is EE2. EE2 is extremely stable, released through most wastewater treatment facilities in an active form, and is highly potent when compared with native fish estrogens (Thorpe et al. 2003). It is widespread in surface waters with detectable concentrations ranging from 0.05 to 831 ng EE2/l (Ternes et al. 1999, Baronti et al. 2000, Huang & Sedlak 2001, Kolpin et al. 2002).
Laboratory exposures of fishes to EE2 have documented numerous reproductive effects. A common experimental endpoint used is the assessment of reproductive success through natural spawning. Even though this endpoint shows that EE2 significantly affects adults, it does not contribute any insight into the mechanism of action regarding effects on gametes for the next generation. To establish an effect of EE2 on male gametes, Schultz et al. (2003) exposed sexually maturing male rainbow trout and utilized an in vitro fertilization approach with eggs from unexposed females to assess embryonic development. Using this method, they reported decreased embryonic survival with sperm from males exposed to concentrations as low as 10 ng EE2/l. Using in vitro fertilization trials when compared with natural spawning, this experimental design uniquely separated adult and gamete-specific EE2 effects, establishing an embryonic survival effect on offspring, not previously shown in fishes.
The decreased embryonic survival reported by Schultz et al. (2003) was assessed at 28 days post-fertilization (dpf), a time point when embryonic development in rainbow trout is nearly complete. While the presence of a significant effect on the male gamete is evident, questions regarding the actual timing of developmental interference remain unanswered. A range of possibilities exists to explain these results, from a failure at fertilization to some defect occurring during embryonic development due to influences of the paternal genome. In addition, the timing of male EE2 exposure is also a variable requiring investigation. To determine the EE2-induced mechanism of action for reduced embryonic survival, the objectives of this study were: (1) to expose male parents at two different points during reproductive development, (2) to track embryonic development from fertilization onward in families derived from male rainbow trout exposed to different concentrations of EE2, and (3) to correlate embryo mortality patterns observed in offspring with reproductive hormones of the pituitary–gonad axis in the paternal parent.
Results
Waterborne and blood plasma EE2 concentrations
The nominal and measured water levels of EE2 during the 1800 and 6700°d experiments are shown in Table 1. Measured EE2 levels were within 60–100% of nominal values for all tested samples. Mean values of measured blood plasma concentrations of EE2 in males from the 6700°d experiment after 56 days were 0.0, 0.55, 2.83, 36.56 ng EE2/ml in relation to nominal concentrations of 0, 1, 10, and 100 ng EE2/l exposures respectively (Fig. 1).
Table 1.
Nominal and measured waterborne 17α-ethynylestradiol (EE2) concentrations (ng EE2/l) in exposure tanks containing male rainbow trout that were 1800 degree days (°d) or 6700°d in age.
| Experiment | Nominal exposure levels | Measured exposure levels (mean±S.D.) | Number of measurements (n) |
|---|---|---|---|
| 6700°d | 100 | 64.6±20 | 11 |
| 100 | 54.7±12 | 11 | |
| 10 | 6.4±2.6 | 5 | |
| 10 | 9.3±4.4 | 4 | |
| 1 | 0.79 | 1 | |
| 1 | 0.84 | 1 | |
| 1800°d | 100 | 124.6±7.4 | 4 |
| 100 | 93.7±6.0 | 4 |
Figure 1.
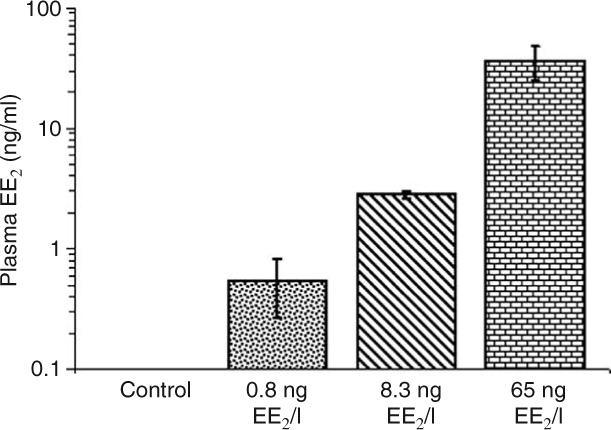
Blood plasma concentrations of EE2 in male rainbow trout after 56-day exposure to different EE2 levels. The bars represent a mean±s.d. (n=3–7). Note that y-axis is on log scale.
In vitro fertilization trials
Sperm per milliliter of semen ranged from 1.16–8.93 billion/ml with means of 4.11, 3.46, 4.31, and 4.99 billion/ml for the control, 0.8, 8.3, and 65 ng EE2/l respectively in the 6700°d experiment. In the 1800°d experiment, sperms per milliliter of semen were 3.40 and 2.83 billion/ml for control and EE2 exposed treatments respectively. There were no significant differences in sperm concentrations between treatments in either experiment.
For the 6700°d experiment, one control group semen sample was inadvertently contaminated with water during collection, which prevented any fertilization in this sample. Embryonic development was therefore assessed on 39 out of 40 individuals sampled. Initial statistical analysis indicated that no tank effects were present for any developmental time points resulting in the ability to collapse the original statistical model to: yijk = μ + αi + eij, where αi represents treatment effects of EE2 and eij the random error. The collapsed model indicated that significant differences were present between all EE2 treatment groups and the control at the 19 dpf developmental time point, eye pigmentation. Analyses of the three earlier developmental time points, 0.5, 2.5, and 9 dpf, revealed significant differences between the 65 ng EE2/l treatment and the control, with no significant differences present between the 0.8 and 8.3 ng EE2/l treatments and the control (Fig. 2). No significant tank or treatment effects on embryonic survival at any developmental time point in the 1800°d experiment were detected (Fig. 3).
Figure 2.
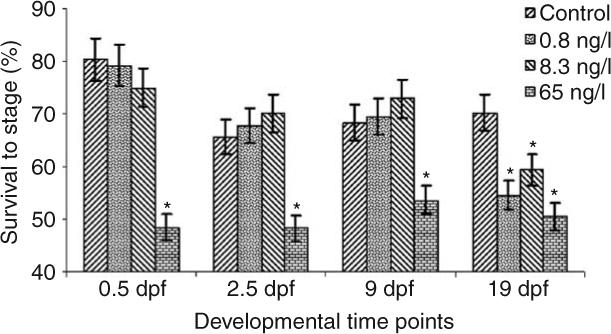
Embryonic survival through 19 days of development post-fertilization for offspring of 6700°d EE2 exposed male rainbow trout. The bars represent a mean±s.d; asterisks indicate significant differences between control and treatment group(s) at P<0.05.
Figure 3.
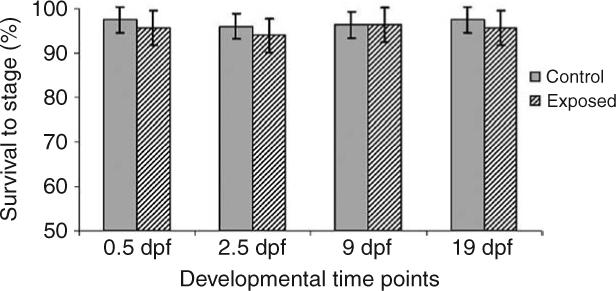
Embryonic survival through 19 days of development post-fertilization for embryos of 1800°d EE2 exposed male rainbow trout. Bars represent a mean±1 s.d.
Vitellogenin and hormone levels
In the 6700°d experiment, plasma vitellogenin was non-detectable in the control group but detectable in all fish exposed to EE2 and exhibited a positive dose–response with increasing EE2 levels. Mean plasma vitellogenin levels were 0.027, 62, and 154 mg/ml for the 0.8, 8.3, and 65 ng EE2/l treatments respectively. Plasma 11-KT levels were similar in all treatment groups, with the exception of fish exposed to 65 ng EE2/l, where levels were significantly below other groups (Fig. 4). Plasma LH levels displayed a pattern in which a significant increase (Fig. 4) was observed in fish exposed to 65 ng EE2/l when compared with other treatment groups. FSH levels did not differ significantly among treatments with mean levels 0.718, 0.461, 0.782, and 0.996 ng/ml for control, 0.8, 8.3, and 65 ng EE2/l treatments respectively.
Figure 4.
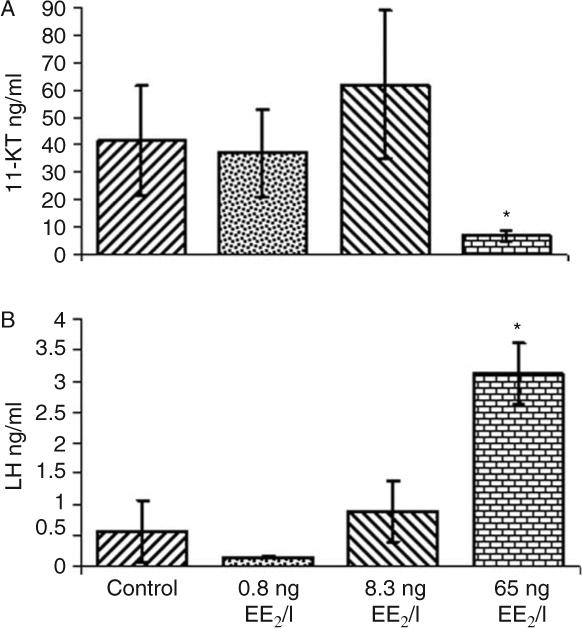
Blood plasma concentrations of (A) 11-ketotestosterone (11-KT) and (B) luteinizing hormone (LH) in 6700°d male rainbow trout after 56 days of exposure to different EE2 levels. The bars represent a mean±s.d. (n=5); the asterisk indicates a significant difference from the control at P>0.05.
Discussion
Effects of environmental estrogens have been noted in a wide range of aquatic species including frogs, salamanders, and fishes (Jones et al. 2000, Gye & Kim 2005, Mosconi et al. 2005). Within fishes, the most commonly reported effect is induction of vitellogenin levels in male and/or juvenile animals (Rotchell & Ostrander 2003). Although these types of studies provide useful information on environmental exposure, relatively few studies go beyond this basic analysis. Notable exceptions exist where studies have examined the role that environmental estrogens have on reproductive success. For example, using various fish model systems, environmental estrogen exposure has been shown to reduce spawning success due to interference with male reproductive behavior (Majewski et al. 2002, Hill & Janz 2003, Martinovic et al. 2007). Schultz et al. (2003) used an in vitro fertilization approach designed to eliminate reproductive behavior effects, specifically analyzing the effect of EE2 on male gametes. This approach revealed the presence of significant sperm-specific decreased survival during early development resulting from EE2 exposure of male parents (Schultz et al. 2003). This reduced embryonic survival effect, affecting unexposed embryo development, represents a considerable challenge for determining a potential mechanism of action. Questions relating to how adult male parental exposure can be transmitted to developing embryos and whether ontogenetic time points of exposure are crucial must be addressed. Here, we illustrate that exposure to EE2 at 1800°d (161 dpf), prior to the initiation of spermatogenesis, has no effect on embryo survival. Either there is no effect of EE2 on these early germ cells or damaged germ cells are removed from the population before fish attain full sexual maturity. We also illustrate that EE2 exposure beginning at 6700°d (587dpf), during late spermatogenesis/spermiation, causes significant reductions in embryonic survival. Therefore, during the final phases of reproductive development in male rainbow trout, testes appears uniquely vulnerable to EE2 at levels as low as ∼1 ng EE2/l. Concentrations of EE2 in this range are environmentally relevant and are therefore a concern for wild fish populations.
This study showed that exposure of sexually maturing male rainbow trout for 56 days to concentrations as low as 0.8 ng EE2/l produce significant reductions in survival to the eye pigmentation stage (19 dpf; Fig. 2). Although these results confirm reduced survival to the eye pigmentation stage previously observed (Schultz et al. 2003), the inclusion of additional developmental time points (early cleavage, blastula formation, and embryonic keel development) revealed two separate time points at which development and/or fertilization is affected. At 65 ng EE2/l, either fertilization or embryonic development to first cleavage is affected. A determination regarding whether this effect is the result of development or fertilization cannot be made as no reliable method is currently available to specifically determine whether fertilization (at 0 dpf) has occurred. In contrast to the 65 ng EE2/l treatment, the 8.3 and 0.8 ng EE2/l doses did not exhibit significant development differences until much later, after embryonic keel formation (9 dpf). The timing of significant embryo mortality, early with 65 ng EE2/l exposed males and occurring later with 8.3 and 0.8 ng EE2/l exposed males, indicates the possibility of two mechanisms of action dependant on EE2 doses the parents receive during late sexual maturation.
Plasma analyses of three hormones in males from the 6700°d experiment revealed significant effects on 11-KT and LH for the 65 ng EE2/l treatment. EE2 does not appear to significantly affect these hormones in sexually maturing males at concentrations below 8.3 ng EE2/l. In fishes, 11-KT is the major male androgen and has been reported to be crucial for spermatogenesis (Miura & Miura 2003). Reduced levels of plasma 11-KT at the 65 ng EE2/l exposure are likely initiated through a direct action of EE2 on the testis. This reduction may result from a receptor-mediated shunting of hormone precursors from androgens to progestins (Kusakabe et al. 2006) or interference with estrogen receptors on Leydig cells (Bouma & Nagler 2001) that produce 11-KT (Le Gac & Loir 1988). Increased LH levels in the 65 ng EE2/l fish could be related to depressed 11-KT levels through a well-known negative feedback association between LH and 11-KT at the pituitary or hypothalamus. This feedback system increases production of LH in male fishes when 11-KT levels are depressed (Yamaguchi et al. 2005). Alternatively, the increase in LH may be unrelated to 11-KT and result from direct action of EE2 on the pituitary. Evidence exists for multiple estrogen response element-like sequences on the LH promoter of several fish species (Yaron et al. 2001, Melamed et al. 2006) and estrogens do interact with these elements, up-regulating the LH gene (Liu et al. 1995). Therefore, EE2 in male rainbow trout could be binding to estrogen receptors and directly influencing LH transcription in the pituitary leading to elevated plasma levels.
Taken together, late sexual maturation exposure differences exhibited between high (65 ng EE2/l) and low (8.3 and 0.8 ng EE2/l) exposures from the 6700°d experiment in regard to both timing of embryo mortality and plasma hormone levels indicate the likelihood of two different mechanisms of action. Observed plasma vitellogenin and plasma EE2 levels support the fact that all treatment groups received EE2 exposures consistent with intended levels and that each had distinctly different levels of EE2. The high concentration (i.e. 65 ng EE2/l) that produced alterations in plasma hormone levels (abnormally low 11-KTand high LH) may compromise the ability of some sperm to fertilize eggs properly. This potential problem with sperm fertilizing ability could account for the immediate, 0.5 dpf, difference in fertilization and/or early development. The combination of lower 11-KT and/or elevated LH may directly influence the final phase of spermatogenesis and spermiation in male rainbow trout. Not all sperm are affected since no evidence exists for complete reproductive failure, but a significant number may be afflicted. While an endocrine-mediated mechanism is possible for the high-dose treatment, no significant plasma hormone changes were present in fish from treatment groups at lower EE2 concentrations. To explain the observed decreased embryonic survival between 9 and 19 dpf in the 0.8 and 8.3 ng EE2/l treatment groups, an alternative mechanism must be considered. In these two groups, a separate non-endocrine-mediated mechanism could be present. Since embryonic development proceeded normally through 9 dpf, some genomic defect of the sperm DNA is proposed. This hypothesis is consistent with other studies in rainbow trout in which sperm DNA was intentionally damaged (Patton et al. 2007). Two lines of evidence support this: no differences were observed between treatment groups and controls at 0.5 or 9 dpf; and this and previous studies have revealed no variation in sperm number, morphology, or swimming ability following EE2 exposure in sexually mature male rainbow trout (Schultz et al. 2003).
Sperm DNA damage could be occurring through two possible mechanisms: aberrant DNA methylation or meiotic interference during spermatogenesis. Exposures of environmental toxicants have been shown to reduce fertility in rats through a process of aberrant DNA methylation of the germ line (Anway et al. 2005). These incorrect DNA methylation patterns were shown to result in heritable defects affecting progeny fertility over multiple generations. Using medaka, Contractor et al. (2004) illustrated that EE2 is capable of inducing methylation changes to the estrogen receptor and aromatase genes, indicating the possibility that EE2-induced DNA methylation changes may be responsible for reduced embryo survival in our study. Alternatively, meiosis during spermatogenesis may be affected. The fact that treatment of male rainbow trout at 1800°d, prior to the initiation of spermatogenesis (i.e. meiosis), did not result in reduced fertility/development supports this hypothesis as well as research in other vertebrates. Bisphenol-A, a less potent environmental estrogen than EE2, has been shown to affect meiosis in female mice resulting in the production of aneuploid gametes (Hunt et al. 2003). The production of such gametes would result in decreased survival of embryos and could account for the observed reduced survival after 9 dpf. Since both mechanisms are possible reasons for reduced embryonic survival, further research is necessary to determine exactly how EE2 exposure of male parents actually reduces embryonic survival of offspring.
Materials and Methods
Research animals and facilities
Fish used in experiments were maintained according to guidelines established by the Institutional Animal Care and Use Committees of Battelle Pacific Northwest National Laboratory, Washington State University (WSU) and the University of Idaho (UI). Male isogenic rainbow trout were produced at the WSU research fish hatchery by fertilizing eggs from a homozygous clonal Oregon State University (OSU) strain female rainbow trout with sperm from a homozygous clonal Arlee strain male rainbow trout. Clonal females and males were originally produced through gynogenesis and androgenesis respectively and are maintained at the WSU research fish hatchery (Parsons & Thorgaard 1985, Young et al. 1996, Young et al. 1998). Offspring resulting from the OSU×Arlee cross, termed clonal hybrids, are genetically identical, greatly reducing levels of phenotypic variation within experimental groups. After fertilization, embryos were placed in a Heath-style fish egg incubator (MariSource, Milton, WA, USA) at 10.5 °C until yolk sac absorption and then transported to Battelle Marine Research Laboratory (MRL) in Sequim, WA, USA. Upon arrival at MRL, fish were 530 degree days (°d=water temperature in °C×number of days) in age. Before and after chemical exposures, trout were housed in groups of 25–75 fish in circular fiberglass tanks ranging in size from 100, 370, or 1400 l depending on fish size. All tanks were maintained with a single-pass flow-through freshwater system. The MRL uses artesian well water (well depth=134 m), pre-aerated before reaching holding tanks at a minimum flow rate of 1 l/min per kg fish. Water quality parameters were routinely measured in holding and treatment tanks, averaging 11.5 °C, >9 mg/l dissolved oxygen, pH 7.9, total alkalinity 200 mg/l (as CaCO3), ammonia <0.05 mg/l, and nitrate–nitrite <0.01 mg/l. Fish were initially fed mashed feed several times a day using automatic feeders and transitioned to soft-moist, pelleted feed of various sizes thereafter (Bio-Oregon Inc., Warrenton, OR, USA). All holding and exposure tanks were maintained under simulated natural photoperiod with graded off- and on-controls.
Exposure trials and sample collections
The EE2 exposures were performed with trout at two different reproductive ontogenetic time points, 1800 and 6700°d. These time points were chosen because they represent initial testicular differentiation from the undifferentiated gonad with the presence of spermatogonia (1800°d; Takashima et al. 1980) and an actively meiotic, mid-spermatogenetic time point with spermatocytes and spermatids predominating the gonad prior to final sexual maturation (6700°d; Kusakabe et al. 2006) in rainbow trout. Degree day exposure times were calculated from 161 and 587 (dpf) using the rearing temperatures in our experimental holding facilities with individual animal weights of ∼10 and 750 g respectively. For exposure treatments, EE2 was delivered using a flow-through system in 370 l tanks from a concentrated stock solution of EE2 prepared in methanol:water (60% v/v) that was slowly added to exposure tanks with a peristaltic pump flow rate of 0.07 ml/min (equals 0.0008% methanol in tanks). Control tanks had only methanol added. For the 1800°d experiment, 60 male rainbow trout were randomly divided into two tank replicates and exposed to a nominal concentration of 100 ng EE2/l or methanol only (solvent control) for 21 days. Following exposures, individuals were transferred to fresh water and allowed to progress normally to sexual maturation when semen samples were collected from five individuals per replicate for a total of ten control and ten exposed males as described below. At 6700°d, 80 male rainbow trout were randomly divided into eight groups of ten fish per tank with two tank replicates per treatment. Individuals were continuously exposed to three treatment levels of EE2 or methanol only (solvent control). Nominal concentration levels of 1, 10, and 100 ng EE2/l were employed. Water and stock solution in-flow rates for all tanks in each treatment were monitored daily. Measured EE2 concentrations were monitored every 7 days for the 1800°d experiment and every 7–14 days for the 10 and 100 ng EE2/l treatments and once for the 1 ng EE2/l treatment for the 6700°d experiment. The 6700°d experiment continued for 56 days after which five randomly selected individuals per tank and ten fish per treatment (40 fish total) were sampled. All fish were anesthetized using buffered 0.25 g/l MS-222 (Argent, Redmond, WA, USA). Individual semen samples from each anesthetized fish were collected by manual expression directly into sterile plastic bags (Whirl-Pak, NASCO, Fort Atkinson, WI, USA) and placed on ice.
For the 6700°d experiment, following semen collection, blood samples were obtained from anesthetized fish using 5 ml heparinized syringes with 21 gauge needles. Blood was drawn from the caudal vein, inverted ten times ensuring proper mixture of heparin, and placed on ice prior to plasma separation by centrifugation. To avoid hemolysis, blood samples remained on ice no longer than 10 min before centrifugation. Plasma was removed after centrifugation and transferred to 1.5 ml microcentrifuge tubes before being placed at –20 °C for storage. After blood collection, fish were euthanized and total weight was obtained.
Determination of EE2 in water and plasma
Water and plasma samples were analyzed for EE2 by GC–MS. Water samples (0.05–1 l in volume) were fortified with NaCl (5% w/v) and extracted with 75 ml methyl-tert-butyl-ether (MTBE) per 1 l sample. Plasma samples (0.2 ml) were mixed with an equal volume of saturated aqueous NaCl solution and extracted with 1 ml MTBE. The MTBE fractions were removed, evaporated under N2, and subsequently derivatized with N-methyl-N-trimethylsilyl-trifluoroacetamide essentially as described in Schultz et al. (2001) except that 3d-estradiol was added as an internal standard in extractions. The GC–MS was operated in selected ion monitoring mode with m/z 419 and m/z 425 ions used for 3d-estradiol and EE2 quantification. EE2 recovery from fortified water standards and blood plasma always exceeded 95%.
In vitro fertilization protocol
Semen from both the 1800 and 6700°d experiments were transported to the UI on ice. For in vitro fertilization trials, both experiments were fertilized using the same batch of pooled, outbred rainbow trout eggs purchased from TroutLodge Inc. (Sumner, WA, USA). Sperm number was quantified using a hemocytometer to determine the number of sperm per microliter of semen. For in vitro fertilization, semen was diluted using immobilizing medium (80 mM NaCl, 40 mM KCl, 0.1 mM CaCl2, 30 mM Tris–HCl, pH 9.2, Cosson et al. 1999) and added to ∼150 unfertilized rainbow trout eggs to produce a sperm:egg ratio of 300 000:1. After the gametes were combined, a small amount (10 ml) of chilled, sperm activation medium (125 mM NaCl, 0.1 mM CaCl2, 30mM Tris–HCl, pH 9.2, Cosson et al. 1999) was added to initiate sperm motility. The gamete mixture was then gently swirled and allowed to stand for 5 min completing fertilization. After 5 min, fertilized eggs were rinsed using cold dechlorinated tap water thrice and placed in Heath incubators. Following fertilization, four developmental time points were assessed: 0.5, 2.5, 9, and 19 dpf, representing early cleavage, raised blastula, embryonic keel, and eye pigmentation stages respectively (Ballard 1973). At each of the first three time points, 25 randomly selected eggs were removed, fixed in Stockard's solution (Ballard 1973), and developmental success gauged as described in Stoddard et al. (2005). The last time point (eye pigmentation) was visually assessed on remaining live embryos.
Plasma vitellogenin and hormone analysis
Plasma vitellogenin levels were determined in samples (freshly thawed on crushed ice) using a rainbow trout vitellogenin ELISA kit (Cayman Chemical, Ann Arbor, MI, USA). Samples were prepared and analyzed as described by the kit assay procedure. The androgen 11-ketotestosterone (11-KT) was determined in freshly thawed plasma samples using an 11-KT ELISA as described by Cuisset et al. (1994). The gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were determined using salmonid RIAs as described by Swanson et al. (1989).
Statistical analysis
Embryonic development data were statistical analyzed using a completely randomized design. Fertilization percentages were analyzed after performing an arcsine transformation to normalize values, while plasma vitellogenin, plasma EE2, and hormone data were analyzed without transformation. The linear model for all analyses was yijk = μ + αi + βj + (αβ)ij + eijk, where αi represents EE2 treatment effects, βj tank effects, (αβ)ij interaction effects, and eijk the random error. ANOVA was performed to determine whether significant differences were present between treatments using PROC GLM in SAS/STAT (SAS Institute, Cary, NC, USA). When indicated by ANOVA, Fisher's protected LSD was performed to determine which treatments differed significantly from one another.
Acknowledgements
The authors would like to thank Mr Steve Patton and Dr Tim Cavileer for their assistance with sample collection, and Dr Sean Lema and Dr Penny Swanson for performing plasma hormone analyses. This research was supported by the National Institute of Environmental Health Sciences grant ES012446. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Anway M, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch GC, Mackenzie CA, Metcalfe CD. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17 alpha-ethinylestradiol. Environmental Toxicology and Chemistry. 2004;23:782–791. doi: 10.1897/02-539. [DOI] [PubMed] [Google Scholar]
- Ballard WW. Normal embryonic stages for salmonid fishes, based on Salmo gairdneri Richardson and Salvelinus fontinalis (Mitchell). Journal of Experimental Zoology. 1973;184:7–26. [Google Scholar]
- Baronti C, Curini R, D'Ascenzo G, Di Corcia A, Gentili A, Samperi R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environmental Science & Technology. 2000;34:5059–5066. [Google Scholar]
- Van den Belt K, Verheyen R, Witters H. Reproductive effects of ethynylestradiol and 4t-octylphenol on the zebrafish (Danio rerio). Archives of Environmental Contamination and Toxicology. 2001;41:458–467. doi: 10.1007/s002440010272. [DOI] [PubMed] [Google Scholar]
- Van den Belt K, Verheyen R, Witters H. Effects of 17 alpha-ethynylestradiol in a partial life-cycle test with zebrafish (Danio rerio): effects on growth, gonads and female reproductive success. Science of the Total Environment. 2003;309:127–137. doi: 10.1016/S0048-9697(03)00046-9. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Berg C, Holm L, Brandt I, Ridderstrale Y, Brunstrom B. Defective reproductive organ morphology and function in domestic rooster embryonically exposed to o,p'-DDT or ethynylestradiol. Biology of Reproduction. 2006;74:481–486. doi: 10.1095/biolreprod.105.045104. [DOI] [PubMed] [Google Scholar]
- Bouma J, Nagler JJ. Estrogen receptor-a protein localization in the testis of the rainbow trout (Oncorhynchus mykiss) during different stages of the reproductive cycle. Biology of Reproduction. 2001;65:60–65. doi: 10.1095/biolreprod65.1.60. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Fernandez MP, Royston S, Smith JL, van Poppelen P, Ikonomou MG, Devlin RH. Male coho salmon (Oncorhynchus kisutch) exposed to a time-course of urban sewage effluent exhibit a sporadic low incidence of sex reversal and intersex. Water Quality Research Journal of Canada. 2006;41:235–243. [Google Scholar]
- Contractor RG, Foran CM, Li SF, Willett KL. Evidence of gender- and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. Journal of Toxicology and Environmental Health. Part A. 2004;67:1–22. doi: 10.1080/15287390490253633. [DOI] [PubMed] [Google Scholar]
- Cosson J, Billard R, Cibert C, Dreanno C, Suquet M. Ionic factors regulating the motility of fish sperm. In: Gagnon C, editor. The Male Gamete: From Basic Science to Clinical Applications. Cache River Press; Vienna, IL: 1999. pp. 161–186. [Google Scholar]
- Cuisset B, Pradelles P, Kime DE, Kuhn ER, Babin P, Le Menn F. Enzyme immunoassay for 11-ketotestosterone using acetylcholinesterase as label: application to the measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comparative Biochemistry and Physiology. Part C, Toxicology & Pharmacology. 1994;108:229–241. [Google Scholar]
- Furuya M, Adachi K, Kuwahara S, Ogawa K, Tsukamoto Y. Inhibition of male chick phenotypes and spermatogenesis by Bisphenol-A. Life Sciences. 2006;78:1767–1776. doi: 10.1016/j.lfs.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Le Gac F, Loir M. Control of testis function in fish: in vitro studies of gonadotropic regulation in the trout (Salmo gairdneri). Reproduction, Nutrition, Development. 1988;28:1031–1046. [Google Scholar]
- Goksoyr A. Endocrine disruptors in the marine environment: mechanisms of toxicity and their influence on reproductive processes in fish. Journal of Toxicology and Environmental Health, Part A: Current Issues. 2006;69:175–184. doi: 10.1080/15287390500259483. [DOI] [PubMed] [Google Scholar]
- Gye MC, Kim DH. Bisphenol A induces hepatic vitellogenin mRNA in male Bombina orientalis. Bulletin of Environmental Contamination and Toxicology. 2005;75:1–6. doi: 10.1007/s00128-005-0710-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Bessho H, Hara A, Nakamura M, Iguchi T, Fujita K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectes yokohamae) from Tokoyo Bay, Japan. Marine Environmental Research. 2000;49:37–53. doi: 10.1016/s0141-1136(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Huang CH, Sedlak DL. Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environmental Toxicology and Chemistry. 2001;20:133–139. [PubMed] [Google Scholar]
- Hill RL, Jr, Janz DM. Developmental extrogenic exposure in zebrafish (Danio rerio): I. effects on sex ratio and breeding success. Aquatic Toxicology. 2003;63:417–429. doi: 10.1016/s0166-445x(02)00207-2. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Current Biology. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- Ingerslev F, Vaclavik E, Halling-Sorensen B. Pharmaceuticals and personal care products: a source of endocrine disruption in the environment? Pure and Applied Chemistry. 2003;75:1881–1893. [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environmental Science & Technology. 1998;32:2498–2506. [Google Scholar]
- Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Baunbeck T, Turner AP, Tyler CR. Comparative response of mollusks and fish to environmental estrogens and an estrogenic effluent. Aquatic Toxicology. 2003;65:205–220. doi: 10.1016/s0166-445x(03)00134-6. [DOI] [PubMed] [Google Scholar]
- Jones PD, De Coen WM, Tremblay L, Giesy JP. Vitellogenin as a biomarker for environmental estrogens. Water Science and Technology : A Journal of the International Association on Water Pollution Research. 2000;42:1–14. [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999−2000: a national reconnaissance. Environmental Science & Technology. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kusakabe M, Nakamura I, Evans J, Swanson P, Young G. Changes in mRNAs encoding steroidogenic acute regulatory protein, steroidogenic enzymes and receptors for gonadotropins during spermatogenesis in rainbow trout testes. Journal of Endocrinology. 2006;189:541–554. doi: 10.1677/joe.1.06684. [DOI] [PubMed] [Google Scholar]
- Liney KE, Jobling S, Shears JA, Simpson P, Tyler CR. Assessing the sensitivity of different life stages for sexual disruption in Roach (Rutilus rutilus) exposed to effluents from wastewater treatment works. Environmental Health Perspectives. 2005;113:1299–1307. doi: 10.1289/ehp.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xiong F, Hew CL. Functional analysis of estrogen-responsive elements in chinook salmon (Oncorhynchus tschawytscha) gonadotropin II beta subunit gene. Endocrinology. 1995;136:3486–3493. doi: 10.1210/endo.136.8.7628385. [DOI] [PubMed] [Google Scholar]
- Ma RS, Sassoon DA. PCBs exert an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract. Environmental Health Perspectives. 2006;114:898–904. doi: 10.1289/ehp.8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski AR, Blanchfield PJ, Palace VP, Wautier K. Waterborne 17(alpha-ethynylestradiol affects aggressive behaviour of male fathead minnows (Pimephales promelas) under artificial spawning conditions. Water Quality Research Journal of Canada. 2002;37:697–710. [Google Scholar]
- Martinovic D, Hogarth WT, Jones RE, Sorensen PW. Environmental estrogens suppress hormones, behavior, and reproductive fitness in male fathead minnows. Environmental Toxicology and Chemistry. 2007;26:271–278. doi: 10.1897/06-065r.1. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Arnold D, Johnson AC, Pepper TJ, Pottinger TG, Pullman KGT. Contamination of headwater streams in the United Kingdom by oestrogenic hormones from livestock farms. Science of the Total Environment. 2006;367:616–630. doi: 10.1016/j.scitotenv.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Melamed P, Zhu Y, Tan SH, Xie M, Koh M. Gonadotropin-releasing hormone activation of c-jun, but not early growth response factor-1, stimulates transcription of a luteinizing hormone beta-subunit gene. Endocrinology. 2006;147:3598–3605. doi: 10.1210/en.2006-0022. [DOI] [PubMed] [Google Scholar]
- Mills LJ, Chichester C. Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Science of the Total Environment. 2005;343:1–34. doi: 10.1016/j.scitotenv.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Miura T, Miura CI. Molecular control mechanisms of fish spermatogenesis. Fish Physiology and Biochemistry. 2003;28:181–186. [Google Scholar]
- Mosconi G, Di Rosa I, Bucci S, Morosi L, Franzoli MF, Polzonetti-Magni AM, Pascolini R. Plasma sex steroid and thyroid hormones profile in male water frogs of the Rana esculenta complex from agricultural and pristine areas. General and Comparative Endocrinology. 2005;142:318–324. doi: 10.1016/j.ygcen.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nash JP, Kime DE, Van der Ven LTM, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environmental Health Perspectives. 2004;112:1725–1733. doi: 10.1289/ehp.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego R, Burgos A, Moraga-Cid G, Inzunza B, Gonzalez M, Valenzuela A, Barra R, Gavilan JE. Effects of pulp and paper mill discharges on caged rainbow trout (Oncorhynchus mykiss): Biomarker responses along a pollution gradient in the Biobio River, Chile. Environmental Toxicology and Chemistry. 2006;25:2280–2287. doi: 10.1897/05-385r.1. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Thorgaard GH. Production of androgenic diploid rainbow trout. Journal of Heredity. 1985;76:177–181. doi: 10.1093/oxfordjournals.jhered.a110060. [DOI] [PubMed] [Google Scholar]
- Patton SJ, Kane SL, Wheeler PA, Thorgaard GH. Maternal and paternal influence on early embryonic survival of androgenetic rainbow trout (Oncorhynchus mykiss): implications for measuring egg quality. Aquaculture. 2007;263:26–34. [Google Scholar]
- Rempel MA, Reyes J, Steinert S, Hwang W, Armstrong J, Sakamoto K, Kelley K, Schlenk D. Evaluation of relationships between reproductive metrics, gender and vitellogenin expression in demersal flatfish collected near the municipal wastewater outfall of Orange County, California, USA. Aquatic Toxicology. 2006;77:241–249. doi: 10.1016/j.aquatox.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Rotchell JM, Ostrander GK. Molecular markers of endocrine disruption in aquatic organisms. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2003;6:453–495. doi: 10.1080/10937400306476. [DOI] [PubMed] [Google Scholar]
- Scholz S, Gutzeit HO. 17-alpha-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquatic Toxicology. 2000;50:363–373. doi: 10.1016/s0166-445x(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Schultz IR, Orner G, Merdink JL, Skillman A. Dose-response relationships and pharmacokinetics of vitellogenin in rainbow trout after intravascular administration of 17 alpha-ethynylestradiol. Aquatic Toxicology. 2001;51:305–318. doi: 10.1016/s0166-445x(00)00118-1. [DOI] [PubMed] [Google Scholar]
- Schultz IR, Skillman A, Nicolas J, Cyr DG, Nagler JJ. Short-term exposure to 17α-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry. 2003;22:1272–1280. [PubMed] [Google Scholar]
- Stoddard JW, Parsons JE, Nagler JJ. Early onset of embryonic mortality in sub-fertile families of rainbow trout (Oncorhynchus mykiss). Reproduction, Fertility and Development. 2005;17:785–790. doi: 10.1071/rd05087. [DOI] [PubMed] [Google Scholar]
- Swanson P, Bernard M, Nozaki M, Suzuki K, Kawauchi H, Dickhoff WW. Gonadotropins I and II in juvenile coho salmon. Fish Physiology and Biochemistry. 1989;7:169–176. doi: 10.1007/BF00004704. [DOI] [PubMed] [Google Scholar]
- Takashima F, Patino R, Nomura M. Histological studies on the sex differentiation in rainbow trout. Bulletin of the Japanese Society of Scientific Fisheries. 1980;46:1317–1322. [Google Scholar]
- Tashiro Y, Takemura A, Fujii H, Takahira K, Nakanishi Y. Livestock wastes as a source of estrogens and their effects on wildlife of Manko tidal flat, Okinawa. Marine Pollution Bulletin. 2003;47:143–147. doi: 10.1016/S0025-326X(03)00053-5. [DOI] [PubMed] [Google Scholar]
- Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants - I. Investigations in Germany, Canada and Brazil. Science of the Total Environment. 1999;225:81–90. doi: 10.1016/s0048-9697(98)00334-9. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, Tyler CR. Relaive potencies and combination effects of steroidal estrogens in fish. Environmental Science & Technology. 2003;37:1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- Versonnen BJ, Janssen CR. Xenoextrogenic effects of ethinylestradiol in zebrafish (Danio rerio). Environmental Toxicology. 2004;19:198–206. doi: 10.1002/tox.20012. [DOI] [PubMed] [Google Scholar]
- Wang HL, Magesan GN, Bolan NS. An overview of the environmental effects of land application of farm effluents. New Zealand Journal of Agricultural Research. 2004;47:389–403. [Google Scholar]
- Woodling JD, Lopez EM, Maldonado TA, Norris DO, Vajda AM. Intersex and other reproductive disruption of fish in wastewater effluent dominated Colorado streams. Comparative Biochemistry and Physiology. Part C, Toxicology & Pharmacology. 2006;144:10–15. doi: 10.1016/j.cbpc.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Gen K, Okuzawa K, Kumakura N, Matsuyama M, Kagawa H. Effects of 11-ketotestosterone and gonadotropin-releasing hormone on follicle-stimulating hormone and luteinizing hormone gene expression in castrated and sham-operated male red seabream Pogrus major. Fisheries Science. 2005;71:1049–1058. [Google Scholar]
- Yaron Z, Gur G, Melamed P, Rosenfeld H, Levavi-Sivan B, Elizur A. Regulation of gonadotropin subunit genes in tilapia. Comparative Biochemistry and Physiology. Part B, Biochemistry and Molecular Biology. 2001;129:498–502. doi: 10.1016/s1096-4959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Young WP, Wheeler PA, Fields RD, Thorgaard GH. DNA fingerprinting confirms isogenecity of androgenetically derived rainbow trout lines. Journal of Heredity. 1996;87:77–81. doi: 10.1093/oxfordjournals.jhered.a022960. [DOI] [PubMed] [Google Scholar]
- Young WP, Wheeler PA, Coryell VH, Keim P, Thorgaard GH. A detailed linkage map of rainbow trout produced using doubled haploids. Genetics. 1998;148:839–850. doi: 10.1093/genetics/148.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]


