Abstract
Glial–neuronal interactions are crucial processes in neuromodulation and synaptic plasticity. The neuregulin 1 family of growth and differentiation factors have been implicated as bidirectional signaling molecules that are involved in mediating some of these interactions. We have shown previously that neuregulin 1 expression is regulated by the gonadal hormones progesterone and 17β-estradiol in the CNS, which might represent a novel, indirect mechanism of the neuromodulatory actions of these gonadal hormones. In the present study, we sought to determine the effects of progesterone and 17β-estradiol on neuregulin 1 expression in rat cortical astrocytes and neurons in vitro. We observed that progesterone increased the expression of neuregulin 1 mRNA and protein in a dose-dependent manner in cultured astrocytes, which was blocked by the progesterone receptor antagonist RU-486. In contrast, 17β-estradiol did not increase either neuregulin 1 mRNA or protein in astrocytes. We observed no effect of either progesterone or 17β-estradiol on neuregulin 1 mRNA and protein in rat cortical neurons in vitro. Finally, we observed that treatment of cortical neurons with recombinant NRG1-β1 caused PSD-95 to localize in puncta similar to that observed following treatment with astrocyte-conditioned medium. These results demonstrate that progesterone regulates neuregulin 1 expression, principally in astrocytes. This might represent a novel mechanism of progesterone-mediated modulation of neurotransmission through the regulation of astrocyte-derived neuregulin 1.
Keywords: Sex hormones, neuregulin, glial activation, PSD-95, NGR1
INTRODUCTION
The hallmark of pathological pain is sensitization of synaptic neurotransmission in the CNS. Previously, this has been attributed to aberrant neuronal activity that involved only neurons of the nociceptive axis in the CNS. However, there is increasing evidence that glial cells have a key role in modulating neuronal processes in the normal and diseased CNS. In particular, glial cells are such active participants in synaptic transmission (Parpura et al., 1994; Pfrieger and Barres, 1997; Araque et al., 2000; Ullian et al., 2001) that the functional unit of neurotransmission should now be considered to include both neuronal and glial components (De Leo et al., 2006). This is particularly true in the case of chronic pain states where glial cells are intimately involved (Garrison et al., 1994; Colburn et al., 1997; DeLeo and Yezierski, 2001; Watkins et al., 2001).
The steroid hormones that are produced primarily by the gonads (i.e. 17β-estradiol, progesterone and testosterone) and their metabolites have profound neuromodulatory activities in the CNS. In the case of nociception, it has been demonstrated that manipulation of hormone levels by removing the gonads alters both basal nociceptive sensitivity and the manifestation of pain-like behaviors in several rodent models of nerve injury (Jordan, 1999; Fillingim and Ness, 2000; Craft et al., 2004). Although general observations about gonadal steroids can be made, the precise nature of how they modulate these behaviors at systems and cellular levels are understood poorly. Furthermore, determining a unifying mechanism of action for gonadal steroids is confounded by the diverse activities of the individual gonadal steroids and their metabolites, which make up the hormonal milieu of the CNS.
The neuregulin 1 (NRG1, also known as NDF, ARIA, GGF and SMDF) family of growth and differentiation factors mediate several crucial functions in the CNS (Buonanno and Fischbach, 2001; Ozaki, 2001; Murphy et al., 2002; Falls, 2003). NRG1 secreted from either neurons or glial cells bind to the extracellular domains of the receptor tyrosine kinases ErbB3 and ErbB4 to initiate a signaling cascade that results in a wide range of biological effects that include modulation of synaptic activity and synaptogenesis (Ozaki et al., 1997; Ozaki et al., 2000; Wolpowitz et al., 2000), induction of neurite arborization (Gerecke et al., 2004), and regulation of the survival and maturation of glial cells (Lemke and Brockes, 1984; Syroid et al., 1996; Adlkofer and Lai, 2000; Chen et al., 2003). Our previous findings indicate that NRG1 is regulated by gonadal steroids, particularly progesterone, in the CNS following nerve root injury. In the present study, we sought to further investigate the role of gonadal steroids in regulating NRG1 expression in astrocytes and neurons in vitro, to elucidate the possible mechanisms involved in gonadal steroid modulation of glial–neuronal signaling.
OBJECTIVE
We sought to determine the role of the gonadal hormones, progesterone and 17β-estradiol, in the regulation of NRG1 expression in astrocytes and neurons in vitro.
METHODS
Reagents
Stock solutions of progesterone, 17β-estradiol, progesterone: BSA:FITC conjugate [progesterone 3-(O-carboxymethyl)oxime:BSA-fluorescein isothiocyanate], RU-486 [mifepristone, 11β-(4-Dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one] and forskolin (7β-acetoxy-1α,6β,9α-trihydroxy-8,13-epoxy-labd-14-en-11-one) (Sigma) were prepared in dimethyl sulfoxide. Stock solutions of dibutyryl-cAMP (db-cAMP; Sigma) and recombinant human NRG1-β1 (rNRG1-β1) EGF domain protein (RandD Systems) were prepared in sterile water.
Primary cell culture
Primary cultures of astrocytes were prepared according to the modified methods of McCarthy et al. (McCarthy and de Vellis, 1980). The cortices of P0–P2 Harlan Sprague Dawley rats (Harlan Indianapolis) were dissected and dissociated using the Worthington Papain Dissociation system (Worthington Biochemical Corporation). The resulting cultures were plated on 75 cm2 flasks in phenol red-free DMEM medium supplemented with charcoal-stripped 10% fetal bovine serum (FBS), 50 μg ml−1 streptomycin, and 50 U ml−1 penicillin. The cells were maintained in this medium for 10−14 days before shaking to remove oligodendrocytes, microglia and neurons. Following this procedure we consistently obtain >98% GFAP-positive astrocytes, determined by immunocytochemistry. The cultures were then trypsinized and replated on either 6-well plates at a density of 200 000 cells well−1 or glass cover-slips in 12-well plates at a density of 50 000 cells well−1 in DMEM supplemented with 10% FBS. All treatments were performed 3 days after replating the cultured astrocytes. The culture medium was replaced with serum-free medium 24 hours before all drug treatments.
Primary E18 cortical neurons were obtained from Genlantis (San Diego) and cultured in Neurobasal medium (Invitrogen GIBCO) supplemented with B-27 (Invitrogen GIBCO), 0.5 mM L-glutamine, 50 μg ml−1 streptomycin and 50 U ml−1 penicillin. The neurons were plated on either poly-D-lysine-coated 6-well plates at a density of 200 000 cells well−1 or poly-D-lysine-coated glass cover-slips in 12-well plates at a density of 20 000 cells well−1. The neurons were treated on the 7th day in culture.
RNA isolation and real-time RT-PCR
Total RNA was isolated directly from the culture dishes using RNeasy Mini kit spin columns (Qiagen) according to the manufacturer's protocol. The purified total RNA was reverse transcribed to cDNA using the Superscipt III First Strand Synthesis kit (Invitrogen). TaqMan primers and FAM (6-carboxyfluorescein)/TAMRA (6-carboxytetramethylrhodamine)-labeled probes for rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and NRG1, and conventional RT-PCR primers for ErbB2, ErbB3 and ErbB4 were designed according to our previously published protocol (Tanga et al., 2004) and are shown in Table 1. The NRG1 primers were designed to amplify a portion of the conserved C-terminal domain and detect most transmembrane NRG1 isoforms, particularly those of the β-subfamily. Primers and probes were synthesized by Applied Biosystems. The qPCR reactions were carried out using the Platinum qPCR Supermix-UDG (Invitrogen) in 96-well plates using the iCycler IQ Multicolor Real-Time PCR detection system (Bio-Rad). The CT values for each qPCR reaction were determined using the iCycler software (Bio-Rad). The relative expression of each of the genes was determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001) using expression of GAPDH as a control, housekeeping gene.
Table 1.
RT-PCR primer and probe sequences
| Gene | Accession # | Primers/Probe | Sequence |
|---|---|---|---|
| GAPDH | NM_01008 | Forward | 5′-CCCCCAATGTATCCGTTGTG-3′ |
| Reverse | 5′-TAGCCCAGGATGCCCTTTAGT-3′ | ||
| Probe | 5′-TGCCGCCTGGAGAAACCTGCC-3′ | ||
| NRG1 | NM_031588 | Forward | 5′-GGCAGTCAGCCCCTTTGTG-3′ |
| Reverse | 5′-TGCAGGGTTGTGATGAAAGGA-3′ | ||
| Probe | 5′-TGCTGCTTGTGACGCCACCAAGG-3′ | ||
| ERBB2 | NM_017003 | Forward | 5′-TCCAGAACCTTCGAATCATTCG-3′ |
| Reverse | 5′-CAATCCACTGCCCAGCTCC-3′ | ||
| ERBB3 | NM_017218 | Forward | 5′-TTCCTGAGGGTGAATCCATCAA-3′ |
| Reverse | 5′-GTACAATGTGGGCATGGTCCA-3′ | ||
| ERBB4 | AF041838 | Forward | 5′-CACGAACACAAGGATAACATCGG-3′ |
| Reverse | 5′-ACATTGCGGGCTGCCAG-3′ |
Immunocytochemistry
The cells were fixed with 4% paraformaldehyde in PBS followed by blocking with 5% FBS/0.1% Triton X-100/PBS for 1 hour at room temperature. The astrocyte coverslips were incubated with a rabbit anti-NRG1 antibody (AB2994, 1:200; Abcam) that recognizes the β isoforms preferentially overnight in blocking buffer at 4°C. The neuron coverslips with incubated with a mouse anti-PSD-95 antibody (MA1−045, 1:200; Affinity BioReagents). All of the coverslips were then washed with PBS/0.1% triton X-100 and incubated with the appropriate Alexa Fluor® 488 (1:250; Invitrogen) secondary antibodies in 3% FBS/0.1% Triton-X 100/PBS for 1 hour at room temperature in the dark. The coverslips were washed three times with PBS, mounted on slides, and protected in Vectashield (Vector Laboratories) containing DAPI. The fluorescent images were captured using a Q-Fire cooled CCD camera (Olympus).
Protein isolation and immunoblotting
Total protein was isolated directly from the culture plates by lysing the cells into Laemmli buffer containing 2% SDS, 10% glycerol, 2% 2-mercaptoethanol, 0.002% bromphenol blue in 75 mM Tris-HCl. The samples were heated to 95°C for 10 min before separating on 10% Tris/Glycine/SDS acrylamide gels (Bio-Rad). The proteins were subsequently transblotted to polyvinylidene difluoride (PVDF; BioRad) membranes and blocked for 1 hour at room temperature in 5% dry milk. The immunoblots were incubated overnight at 4°C with a rabbit anti-NRG1 antibody (AB2994, 1:100; Abcam). After three washes with PBS/0.05% Tween-20 the blots were incubated with a horseradish peroxidase-conjugated goat anti-rabbit antibody (1:3000; Pierce). Protein signal was visualized using the SuperSignal West Femto reagent (Pierce) and detected using the Typhoon Imaging System (GE Healthcare). The immunoblots were washed briefly and then incubated a monoclonal mouse anti-β-actin antibody (1:10 000; Abcam) for 1 hour at room temperature followed by horseradish peroxidase-conjugated goat anti-mouse antibody. β-actin protein was then visualized and detected as above.
RESULTS
Progesterone regulates NRG1 mRNA expression through a cytoplasmic progesterone receptor-dependent mechanism in cultured astrocytes
Rat primary astrocytes were prepared to determine if progesterone directly regulates the expression of NRG1 and ErbB2−4 mRNA in astrocytes. Conventional RT-PCR analysis of untreated astrocytes demonstrated basal expression of NRG1 and ErbB2/ErbB4 in these cultures (Fig. 1A). Conversely, only a minor ErbB3 mRNA signal was detected in these cultured astrocytes. Following application of progesterone to the astrocyte cultures, significant (P<0.01), time-dependent and dose-dependent increases in NRG1 mRNA were observed (Fig. 1B). Maximal induction of NRG1 by 100 nM progesterone was observed 2 hours after treatment and returned to basal levels by 12 hours (Fig. 1C). No further, significant increases in NRG1 mRNA were observed after the application of 10 nM progesterone, which indicates the induction was maximal at that concentration. No significant increase in NRG1 mRNA was observed following the application of 100 nM 17β-estradiol (Fig. 1B). Preincubation with the progesterone receptor antagonist RU-486 (50 nM) before 100 nM progesterone blocked the progesterone-mediated induction of NRG1 mRNA (Fig. 2). Furthermore, treatment with an equivalent concentration of a progesterone:BSA conjugate compound, which is incapable of passive membrane diffusion, did not cause a significant increase in NRG1 mRNA (Fig. 2). These data demonstrate that the progesterone-dependent increase in NRG1 mRNA is dependent on the progesterone receptor. No significant changes in ErbB2−4 mRNA levels were observed at any time point or dose of progesterone in our cultured astrocytes (data not shown).
Fig. 1. Progesterone increases NRG1 mRNA expression in rat astrocytes in culture.
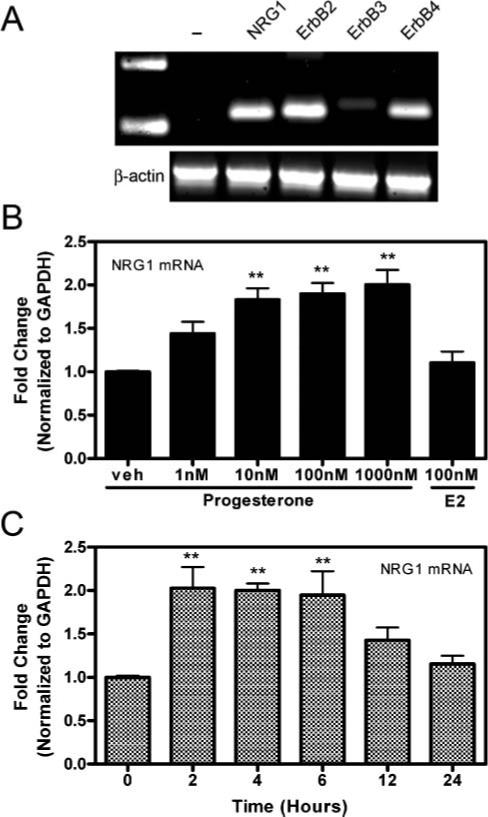
(A) Conventional RT-PCR for NRG1, ErbB2, ErbB3 and ErbB4, including the β-actin housekeeping gene and no NRG1 primer controls (-) in untreated astrocytes in culture. (B) A dose-dependent increase in NRG1 mRNA expression is shown by real-time RT-PCR following 6-hour treatment with 1−1000 nM progesterone but not 100 nM 17β-estradiol (E2) in cultured astrocytes (n=3 at each time point; veh, vehicle). (C) The time-course of progesterone-dependent induction of NRG1 mRNA measured by real-time RT-PCR at 0, 2, 4, 6, 12 and 24 hours after application of 100 nM progesterone to cultured astrocytes (n=3 at each time point). Results are shown as the relative fold-change of the GAPDH-normalized samples compared to the vehicle-treated or 0 hour time-point group. Data are mean±SEM. **, significant increase (P<0.01) in mRNA expression compared with either veh-treated and 0 hour control groups.
Fig. 2. Progesterone-mediated increase in NRG1 mRNA is dependent on cytosolic progesterone receptors.
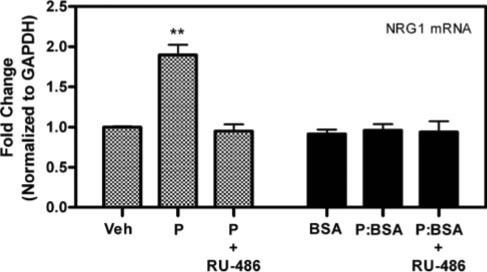
Pretreatment for 30 min with 50 nM of the progesterone receptor antagonist RU-486 blocks the progesterone (P, 100 nM)-mediated increase in NRG1 mRNA expression. Treatment with 100 nM equivalent of a membrane-limited progesterone:BSA conjugate (P:BSA) does not significantly increase NRG1 mRNA expression. Data are the relative fold-change of GAPDH-normalized samples compared to the vehicle-treated (Veh) or BSA groups. Data are mean±SEM. **, significant increase (P<0.01) in mRNA expression compared to Veh controls.
Progesterone regulates NRG1 concentration
Immunocytochemical staining of cultured astrocytes treated with 100 nM progesterone demonstrated an increase in the quantity and intensity of immunoreactive NRG1 compared to control astrocytes following 6 hours of treatment (Fig. 3). This increase in NRG1 immunoreactivity was inhibited by 30-min preincubation with 50 nM RU-486 (Fig. 3D). The cAMP-elevating agent, forskolin, which increases the concentration of NRG1 protein in astrocytes in vitro in a dose-dependent manner (Tokita et al., 2001), was used as a positive control (Fig. 3B). Immunoblot analysis of total NRG1 protein from astrocyte cell lysates demonstrated that a 12-hour treatment with either 1 μM progesterone or 250 μM db-cAMP increased the concentration of a ∼44 kDa isoform of NRG1 (Fig. 4). It has been established previously that multiple isoforms are observed when immunoblotting with antibodies directed towards NRG1 (Raabe et al., 1998). Treatment with 100 nM 17β-estradiol for 12 hours did increase total NRG1 concentration compared with the medium-alone control.
Fig. 3. Progesterone and forskolin regulate NRG1 concentration in cultured astrocytes.
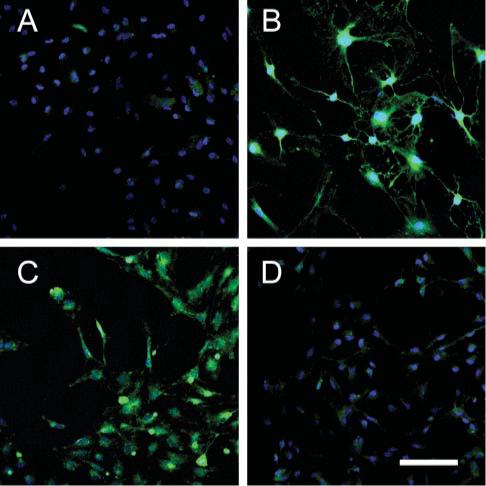
Representative staining with an antibody that preferentially recognizes the β isoforms of NRG1 (green) on astrocytes treated with (A) medium alone, (B) 10 mM forskolin, (C) 100 nM progesterone, and (D) 30-min pretreatment with 50 nM RU-486 followed by 100 nM progesterone for 6 hours. Nuclei are stained with DAPI (blue). Scale bar, 100 μm.
Fig. 4. Progesterone and db-cAMP increase the concentration of NRG1 in cultured astrocytes.
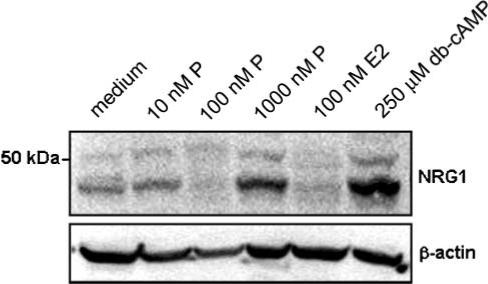
Representative immunoblot of total cellular NRG1 in cultured astrocytes treated with progesterone (P, 10−1000 nM), 17β-estradiol (E2, 100 nM), and medium-alone control. Treatment with db-cAMP is used as a positive control. β-actin is a loading control.
Progesterone does not regulate NRG1 mRNA or protein in neurons in culture
Conventional RT-PCR analysis of rat cortical neurons in culture demonstrated basal expression of NRG1 and ErbB4 in these cultures (Fig. 5A). As with cultured astrocytes, considerably less ErbB3 mRNA was detected. In contrast to astrocyte cultures, no ErbB2 mRNA expression was detected in neurons in culture. No significant changes in NRG1 mRNA expression were observed following treatment of cortical neurons with increasing concentrations of progesterone or with 100 nM 17β-estradiol compared with vehicle-treated neurons (Fig. 5B). Similarly, no significant changes in cellular NRG1 protein were observed following treatment with progesterone, 17β-estradiol, db-cAMP or rNRG1-β1 protein compared with untreated cultured neurons (Fig. 5C).
Fig. 5. NRG1 mRNA and protein is not regulated by progesterone in cultured neurons.
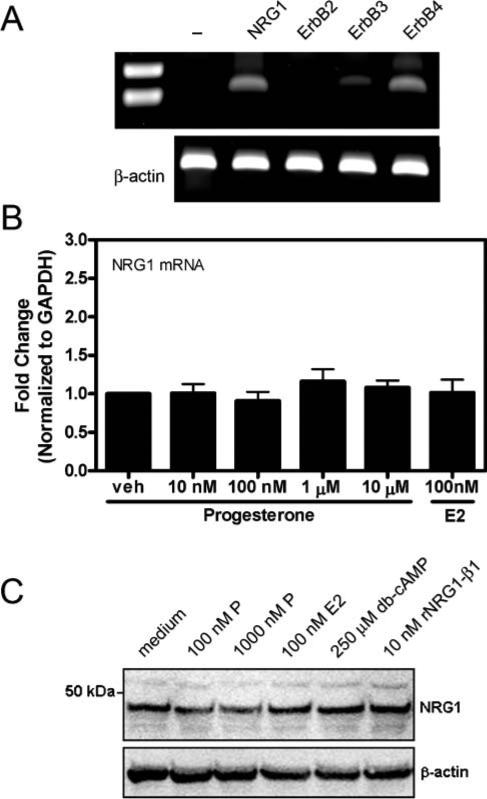
(A) Conventional RT-PCR for NRG1, ErbB2, ErbB3, ErbB4 and β-actin (housekeeping gene), and no-NRG1-primer control (-) in untreated neurons in culture. (B) No significant changes in NRG1 mRNA expression were observed by real-time RT-PCR following 6 hours of treatment with either 10−10 000 nM progesterone or 100 nM 17β-estradiol (E2) (n=3 at each time point). Results are shown as the relative fold-change of the GAPDH-normalized samples compared to vehicle (veh)-treated group. Data are mean±SEM. (C) No significant changes were observed in NRG1 concentration following 6-day treatment with 100−1000 nM progesterone (P), 100 nM E2, 250 μM db-cAMP or 10 nM rNRG1-β1 protein.
Astrocyte-conditioned medium and rNRG1-β1 induce PSD-95 localization in cultured neurons
To examine the possible effects of astrocyte-derived NRG1 on modulation of neuronal synapses, we treated cortical neurons in vitro with either astrocyte-conditioned medium (ACM) or 10 nM rNRG1-β1 protein for 5 days. It has been demonstrated previously that ACM contains soluble factors that modulate synaptogenesis and synaptic activity, measured by the localization of presynaptic and postsynaptic markers (Pfrieger and Barres, 1997; Ullian et al., 2001). Immunocytochemical staining for the postsynaptic density protein PSD-95, a scaffolding protein involved in the clustering of neurotransmitter receptors and downstream signaling molecules in excitatory synapses, demonstrated that ACM increased the intensity of punctate PSD-95 staining (Fig. 6B). Similarly, treatment of cultured neurons with rNRG1-β1 also increased the intensity of punctate staining for PSD-95 to a level similar to that observed in ACM-treated neurons (Fig. 6C). The control neurons that received medium alone showed either few or no PSD-95 puncta (Fig. 6A).
Fig. 6. rNRG1-β1 and ACM induce similar localization of PSD-95 in cultured neurons.
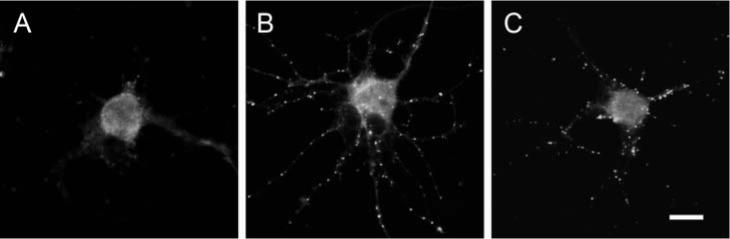
Cortical neurons in culture were treated with (A) medium alone, (B) ACM and (C) 10 nM rNRG1-β1 for 5 days before immunostaining for PSD-95. Both ACM and rNRG1-β1 caused PSD-95 to localize in puncta. Scale bar, 10 μm.
CONCLUSIONS
Progesterone increases the expression of NRG1 mRNA in primary astrocytes in culture via classical progesterone receptors.
Treatment with progesterone, but not 17β-estradiol, increases the amount of total NRG1 protein in astrocytes in culture.
Progesterone does not regulate NRG1 mRNA and protein in primary cultures of neurons.
rNRG1-β1 and ACM induce PSD-95 localization in cultured neurons to similar extents, which indicates that NRG1 acts as a synaptic modulator.
DISCUSSION
We have demonstrated a novel, progesterone-dependent regulation of NRG1 mRNA and protein in astrocytes in vitro. Conversely, we observed no induction of NRG1 mRNA and protein in cortical neurons following treatment with either progesterone or 17β-estradiol. These findings indicate that a progesterone-dependent regulatory system exists in the CNS. It is known that the actions of gonadal steroids are highly tissue-specific and depend on coregulator proteins that act as either positive or negative regulators of steroid hormone-receptor signaling (Khan and Nawaz, 2003; Hall and McDonnell, 2005). Furthermore, it has been demonstrated previously that progesterone regulates the concentration of NRG1 in epithelial cells (Balana et al., 1999) and that NRG1 regulates expression of the progesterone receptor in a reciprocal fashion (Labriola et al., 2003). In this manner, progesterone and NRG1 act together in a feed-forward mechanism that potentiates both progesterone sensitivity and increases the expression of NRG1. These data, together with our findings, indicate that a progesterone–NRG1 regulatory system might also be present in CNS astrocytes. It remains to be determined whether treatment of cultured astrocytes with NRG1 regulates progesterone-receptor expression in a manner similar to epithethial cells.
A growing body of evidence has demonstrated a key role for growth factors and neurotrophic factors such as NRG1 as algogenic mediators in chronic pain states. Astrocytes have been postulated to have a key role in the production and secretion of these growth factors, and have been demonstrated to increase their expression of several growth factors after they are activated in response to CNS injury (Ridet et al., 1997). In this manner, increased local production of NRG1 by astrocytes might simultaneously serve beneficial and detrimental functions in chronic pain states. It is known that neuregulins have major trophic (Oka et al., 2000; Chen et al., 2003) and promyelination functions (Cannella et al., 1998; Michailov et al., 2004; Lemke, 2006) which might protect the damaged CNS while simultaneously sensitizing nociceptive systems.
PSD-95 is a key component in the architecture of excitatory synapses. PSD-95 acts a scaffolding protein that brings together a variety of receptors, signaling molecules and kinases via a special protein-interaction PDZ domain (Hata and Takai, 1999). In particular, PSD-95 has been shown to interact with several subunits of the N-methyl-D-aspartate (NMDA) receptor (Kornau et al., 1995) and might have a key role in clustering these receptors at the post-synaptic membrane to allow efficient crosstalk with downstream signaling molecules. Several studies have shown that PSD-95, which is normally located diffusely in cultured neurons, localizes into distinct puncta in the presence of a synaptogenic signal. A study by Ullian et al. (2001) has shown that medium from astrocytes in culture contains factors that induce PSD-95 localization into puncta as an element of synapse formation in neurons in response to ACM (Pfrieger and Barres, 1997; Ullian et al., 2004). Attempts to isolate individual factor(s) in ACM that promote synapse formation led to the identification of thrombospondin-1 and thrombospondin-2 (Christopherson et al., 2005). Our finding that NRG1-β1 also induces PSD-95 to localize into puncta indicates that this astrocyte-derived factor might also have a role in the astrocyte-mediated modulation of synapses. Injection of NRG1-β1 into the hippocampus regulates synaptic activity transiently, as measured by electrophysiology in vivo (Roysommuti et al., 2003). Further studies investigating the role of NRG1 as a synaptic modulator should uncover the molecular events that mediate this change in synaptic activity in neurons.
In conclusion, we have demonstrated that progesterone, but not 17β-estradiol, regulates the expression of NRG1 mRNA and protein in astrocytes in culture. Conversely, no significant regulation of NRG1 was observed in cultured neurons treated with either progesterone or 17β-estradiol. Finally, we have shown that NRG1-β1 and ACM cause PSD-95 to localize in puncta to a similar extent. These data highlight the importance of steroid-hormone regulation of astrocyte factors that might regulate synaptic function in neurons, and highlight the complex control of glial–neuronal signaling networks.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Ms Kathryn Bercury for her assistance with the astrocyte cultures. This study was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases RO1 AR44757 (J.A.D.).
REFERENCES
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. Journal of Neuroscience. 2000;20:666–763. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balana ME, Lupu R, Labriola L, Charreau EH, Elizalde PV. Interactions between progestins and heregulin (HRG) signaling pathways: HRG acts as mediator of progestins proliferative effects in mouse mammary adenocarcinomas. Oncogene. 1999;18:6370–6379. doi: 10.1038/sj.onc.1203028. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Current Opinion in Neurobiology. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, et al. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proceedings of the National Academy of Sciences of the U.S.A. 1998;95:10100–10105. doi: 10.1073/pnas.95.17.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nature Neuroscience. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. Journal of Neuroimmunology. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. European Journal of Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Experimental Cell Research. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Experimental Neurology. 1994;129:237–243. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Molecular and Cellular Neuroscience. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Molecular Interventions. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- Hata Y, Takai Y. Roles of postsynaptic density-95/synapse-associated protein 90 and its interacting proteins in the organization of synapses. Cellular and Molecular Life Sciences. 1999;56:461–472. doi: 10.1007/s000180050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL. Glia as mediators of steroid hormone action on the nervous system: An overview. Journal of Neurobiology. 1999;40:434–445. doi: 10.1002/(sici)1097-4695(19990915)40:4<434::aid-neu2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Khan OY, Nawaz Z. Nuclear hormone receptor coregulators. Current Opinion in Drug Discovery and Development. 2003;6:692–701. [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, et al. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB2 and mitogen-activated protein kinase activation in breast cancer cells. Molecular and Cellular Biology. 2003;23:1095–1111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. Neuregulin-1 and myelination. Science STKE. 2006;2006:pe11. doi: 10.1126/stke.3252006pe11. [DOI] [PubMed] [Google Scholar]
- Lemke GE, Brockes JP. Identification and purification of glial growth factor. Journal of Neuroscience. 1984;4:75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (Duluth) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. Journal of Cell Biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Murphy S, Krainock R, Tham M. Neuregulin signaling via erbB receptor assemblies in the nervous system. Molecular Neurobiology. 2002;25:67–77. doi: 10.1385/MN:25:1:067. [DOI] [PubMed] [Google Scholar]
- Oka N, Kawasaki T, Matsui M, Tachibana H, Sugita M, Akiguchi I. Neuregulin is associated with nerve regeneration in axonal neuropathies. Neuroreport. 2000;11:3673–3676. doi: 10.1097/00001756-200011270-00017. [DOI] [PubMed] [Google Scholar]
- Ozaki M. Neuregulins and the shaping of synapses. Neuroscientist. 2001;7:146–154. doi: 10.1177/107385840100700209. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Tohyama K, Kishida H, Buonanno A, Yano R, Hashikawa T. Roles of neuregulin in synaptogenesis between mossy fibers and cerebellar granule cells. Journal of Neuroscience Research. 2000;59:612–623. doi: 10.1002/(SICI)1097-4547(20000301)59:5<612::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Raabe TD, Francis A, DeVries GH. Neuregulins in glial cells. Neurochemical Research. 1998;23:311–318. doi: 10.1023/a:1022449231651. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends in Neurosciences. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Carroll SL, Wyss J.M. Neuregulin-1beta modulates in vivo entorhinal-hippocampal synaptic transmission in adult rats. Neuroscience. 2003;121:779–785. doi: 10.1016/s0306-4522(03)00503-7. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee KF, et al. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proceedings of the National Academy of Sciences of the U.S.A. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochemistry International. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, et al. Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. Journal of Neuroscience. 2001;21:1257–1264. doi: 10.1523/JNEUROSCI.21-04-01257.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Molecular and Cellular Neuroscience. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in Neurosciences. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]


