Abstract
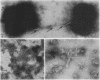
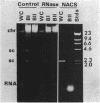
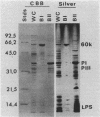
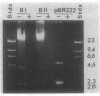
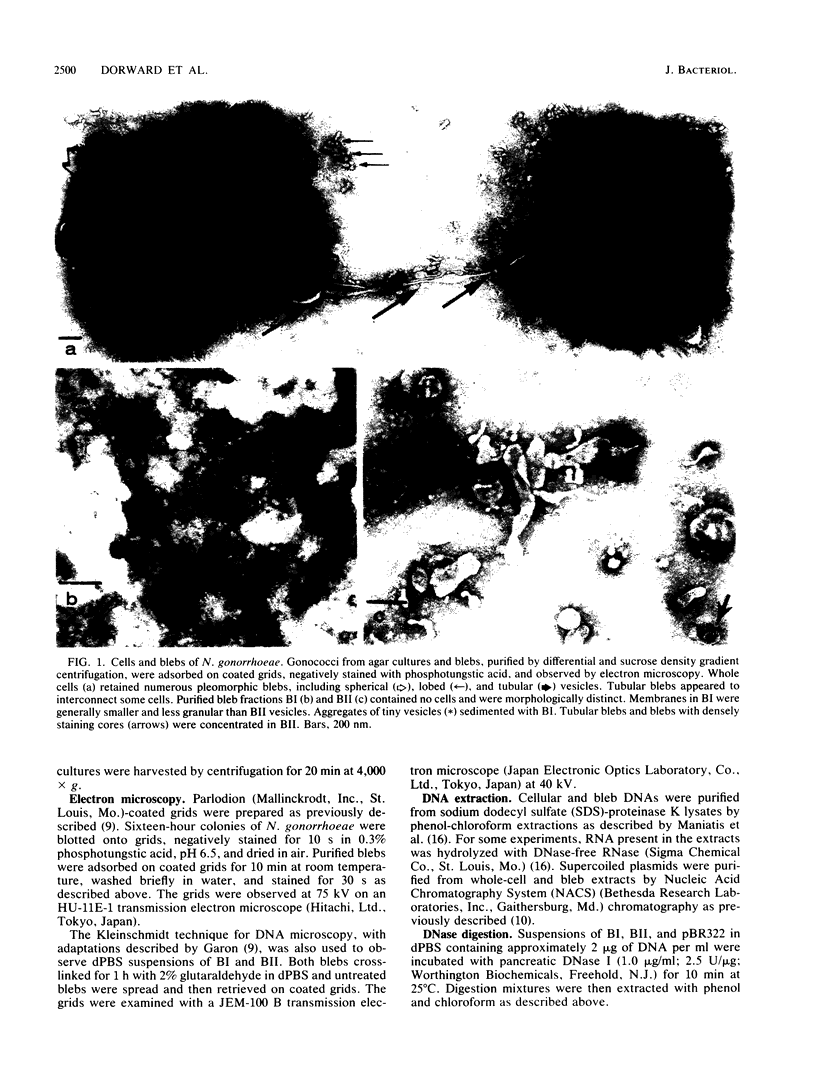
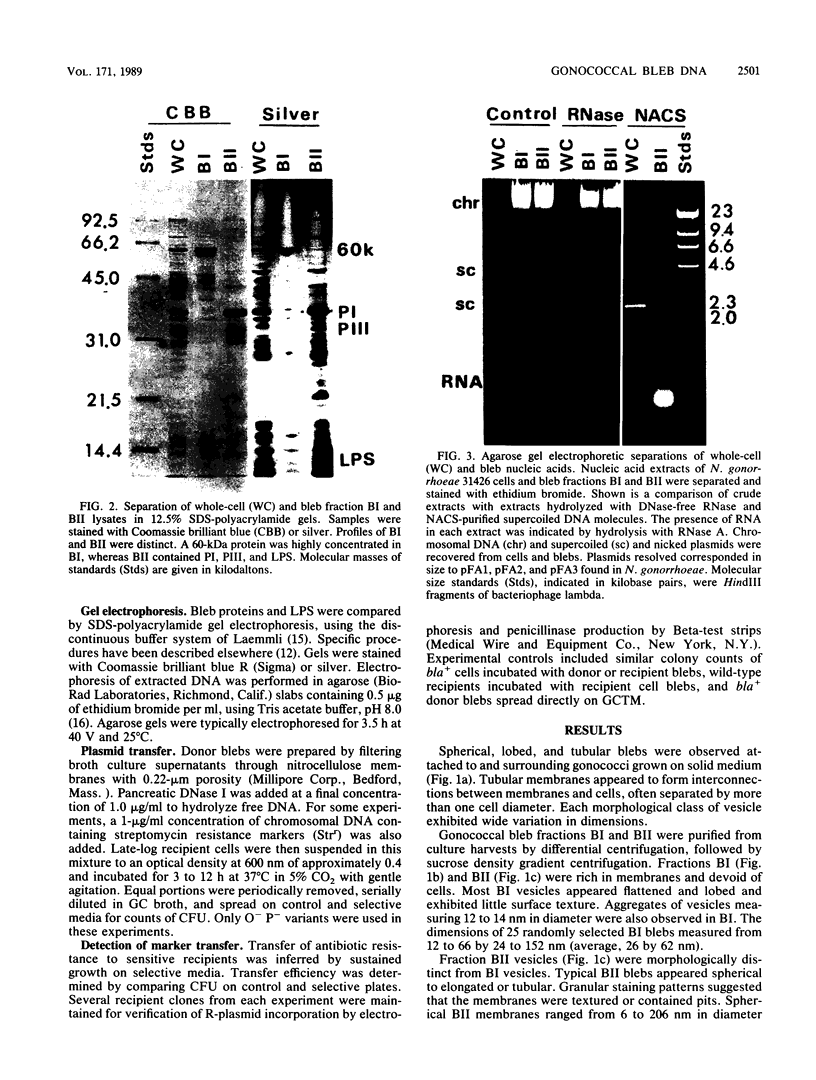
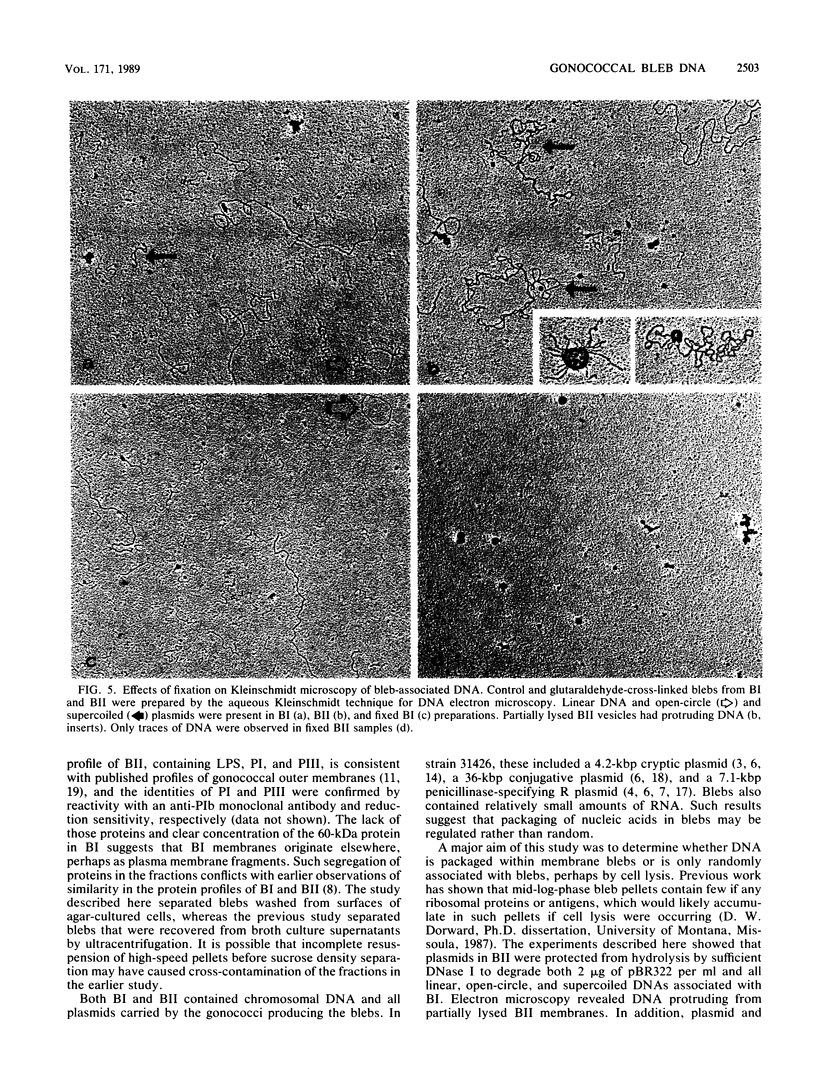
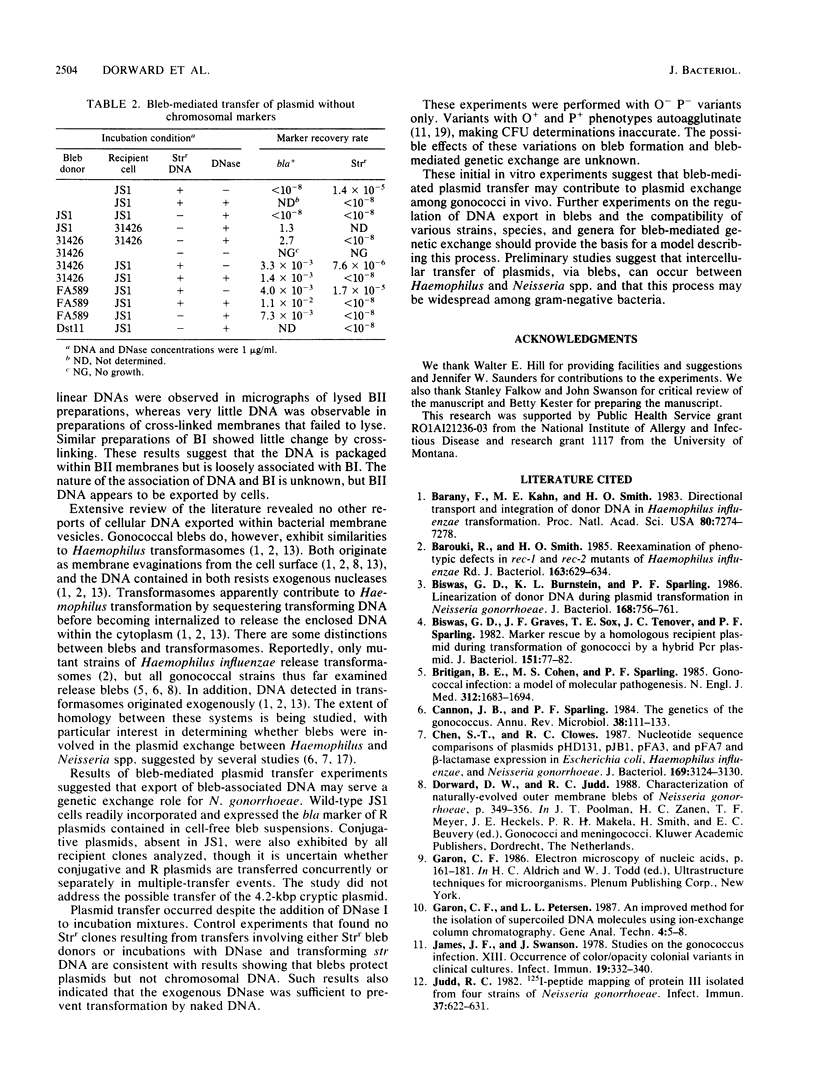
Naturally elaborated membrane bleb material is frequently observed in cultures of Neisseria gonorrhoeae. This material was purified and analyzed for protein, lipopolysaccharide, and nucleic acid content. The electrophoretic protein profiles of two bleb-rich fractions, called BI and BII, were distinct, with only BII containing lipopolysaccharide and outer membrane proteins I and III. Both fractions contained RNA, circular DNA, and linear DNA. Exogenous pancreatic DNase I appeared to hydrolyze all bleb-associated DNA in fraction BI and the linear DNA in fraction BII. The circular DNA molecules associated with fraction BII resisted digestion. Electron microscopy of the bleb fractions verified their DNA content. Fixing blebs with glutaraldehyde before mounting them for microscopy prevented release of internal DNA. Such fixation produced little change in the micrographs of BI; however, only traces of DNA were observed in fixed BII preparations. Incubation of wild-type gonococci in mixtures of DNase and blebs purified from antibiotic-resistant strains resulted in efficient exchange of penicillinase-specifying R plasmids. Recipients incorporated plasmids independently of endogenous and exogenous chromosomal streptomycin resistance markers. These in vitro results suggest that bleb formation by N. gonorrhoeae may serve to transfer plasmids intercellularly in vivo, perhaps constituting a previously unexplored genetic exchange mechanism in these bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Burnstein K. L., Sparling P. F. Linearization of donor DNA during plasmid transformation in Neisseria gonorrhoeae. J Bacteriol. 1986 Nov;168(2):756–761. doi: 10.1128/jb.168.2.756-761.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Graves J. F., Sox T. E., Tenover F. C., Sparling P. F. Marker rescue by a homologous recipient plasmid during transformation of gonococci by a hybrid Pcr plasmid. J Bacteriol. 1982 Jul;151(1):77–82. doi: 10.1128/jb.151.1.77-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Nucleotide sequence comparisons of plasmids pHD131, pJB1, pFA3, and pFA7 and beta-lactamase expression in Escherichia coli, Haemophilus influenzae, and Neisseria gonorrhoeae. J Bacteriol. 1987 Jul;169(7):3124–3130. doi: 10.1128/jb.169.7.3124-3130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Petersen L. L. An improved method for the isolation of supercoiled DNA molecules using ion-exchange column chromatography. Gene Anal Tech. 1987 Jan-Feb;4(1):5–8. doi: 10.1016/0735-0651(87)90006-9. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Barany F., Smith H. O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by a conjugative plasmid of Haemophilus ducreyi. J Bacteriol. 1983 Oct;156(1):437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]