Abstract
In several Escherichia coli K-12 strains grown on a limiting concentration of glucose, isocitrate dehydrogenase (IDH) was inactivated about 90% after cessation of growth upon exhaustion of the glucose. Such inactivation has been previously observed in several E. coli strains but not in E. coli K-12 (unless acetate was added to the bacterial culture when growth ceased). IDH was inactivated 75 to 80% in all E. coli K-12 strains we examined during growth on acetate. The inactivation involved phosphorylation of the enzyme and is considered to be a regulatory mechanism facilitating metabolite flow along the glyoxylate shunt. Phospho-IDH interacted with antibodies to enzymatically active IDH. We have devised a method, based on this immunological cross-reaction, for determining the proportions of active and inactive (phospho-) IDH in cell extracts.
Full text
PDF

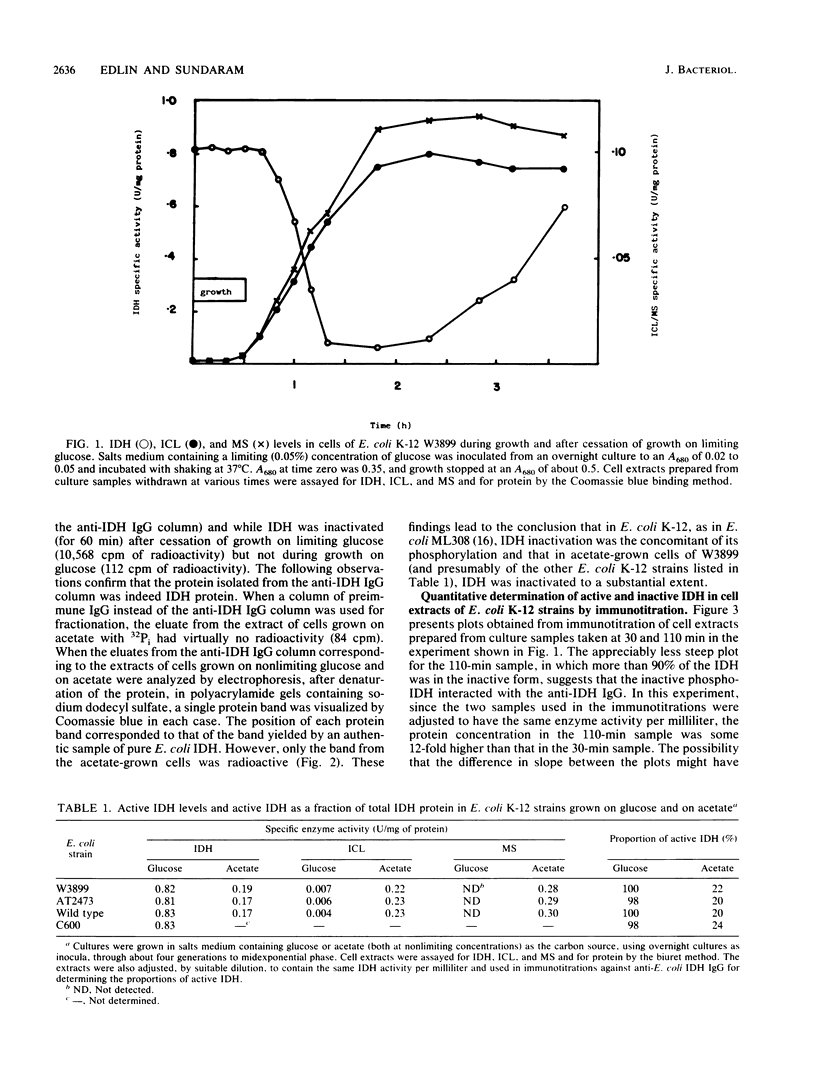
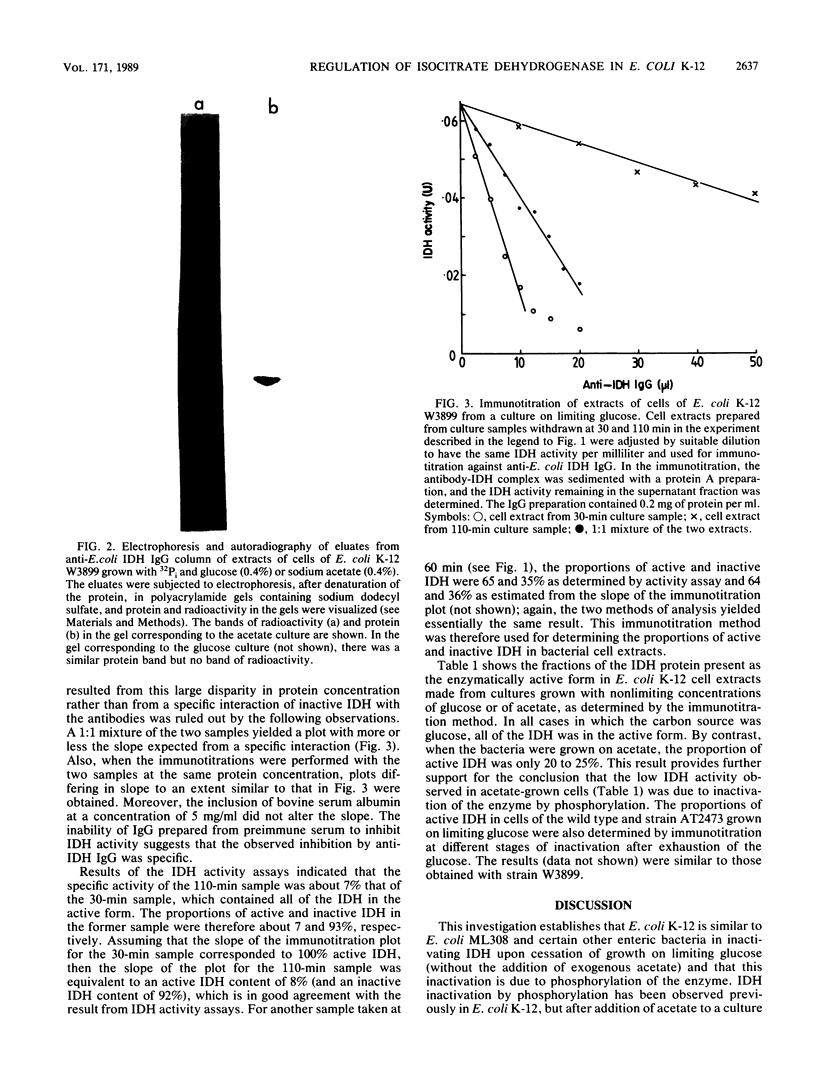

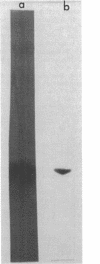
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Holms W. H. Reversible inactivation of the isocitrate dehydrogenase of Escherichia coli ML308 during growth on acetate. J Gen Microbiol. 1975 Mar;87(1):37–51. doi: 10.1099/00221287-87-1-37. [DOI] [PubMed] [Google Scholar]
- Chell R. M., Sundaram T. K. Structural basis of the thermostability of monomeric malate synthase from a thermophilic Bacillus. J Bacteriol. 1978 Aug;135(2):334–341. doi: 10.1128/jb.135.2.334-341.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell R. M., Sundaram T. K., Wilkinson A. E. Isolation and characterization of isocitrate lyase from a thermophilic Bacillus sp. Biochem J. 1978 Jul 1;173(1):165–177. doi: 10.1042/bj1730165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson E. S., Sundaram T. K. Chromosomal location of a gene defining nicotinamide deamidase in Escherichia coli. J Bacteriol. 1970 Mar;101(3):1090–1091. doi: 10.1128/jb.101.3.1090-1091.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Purification and properties of phosphorylated isocitrate dehydrogenase of Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):7915–7920. [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Holms W. H., Nimmo H. G. Reversible inactivation of isocitrate dehydrogenase in Escherichia coli. Biochem Soc Trans. 1982 Oct;10(5):319–320. doi: 10.1042/bst0100319. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Chung T. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem. 1985 Dec 5;260(28):15291–15297. [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983 Sep 22;305(5932):286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Thorsness P. E., Koshland D. E., Jr Compensatory phosphorylation of isocitrate dehydrogenase. A mechanism for adaptation to the intracellular environment. J Biol Chem. 1985 Sep 5;260(19):10563–10568. [PubMed] [Google Scholar]
- Sundaram T. K. Myo-inositol catabolism in Salmonella typhimiurium: enzyme repression dependent on growth history of organism. J Gen Microbiol. 1972 Nov;73(2):209–219. doi: 10.1099/00221287-73-2-209. [DOI] [PubMed] [Google Scholar]
- Sundaram T. K., Wright I. P., Wilkinson A. E. Malate dehydrogenase from thermophilic and mesophilic bacteria. Molecular size, subunit structure, amino acid composition, immunochemical homology, and catalytic activity. Biochemistry. 1980 May 13;19(10):2017–2022. doi: 10.1021/bi00551a002. [DOI] [PubMed] [Google Scholar]