Abstract
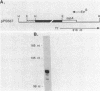
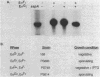
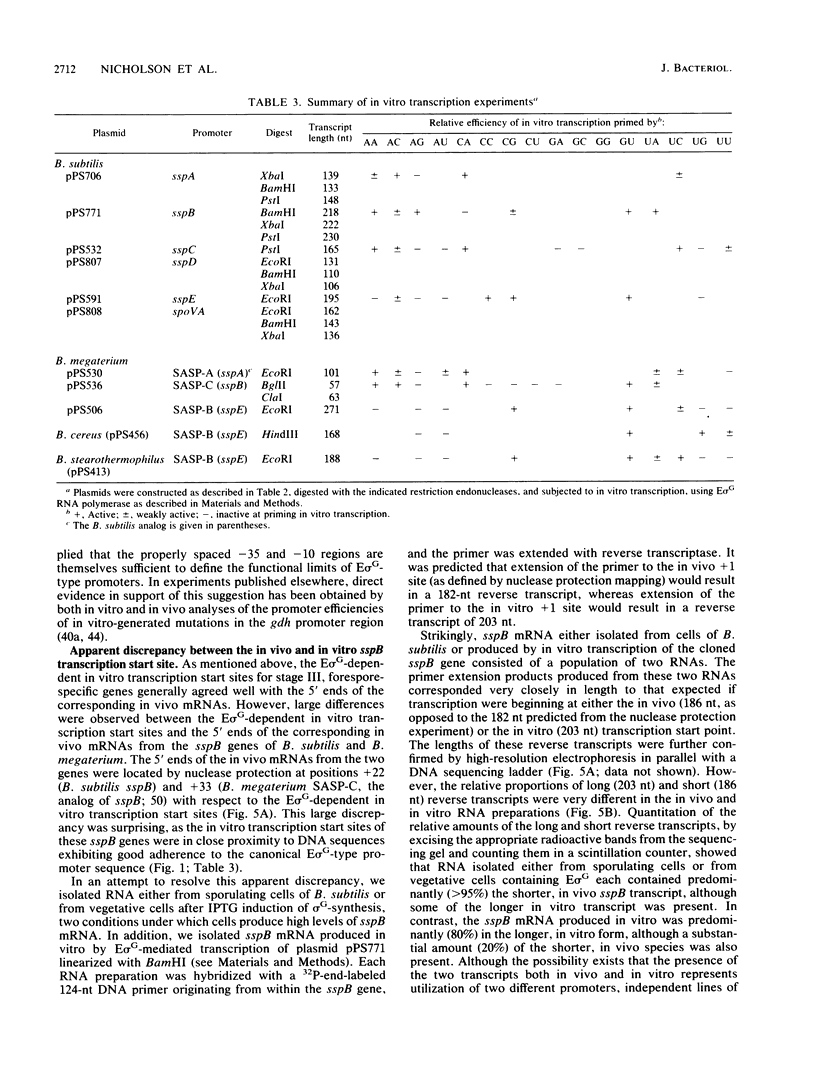
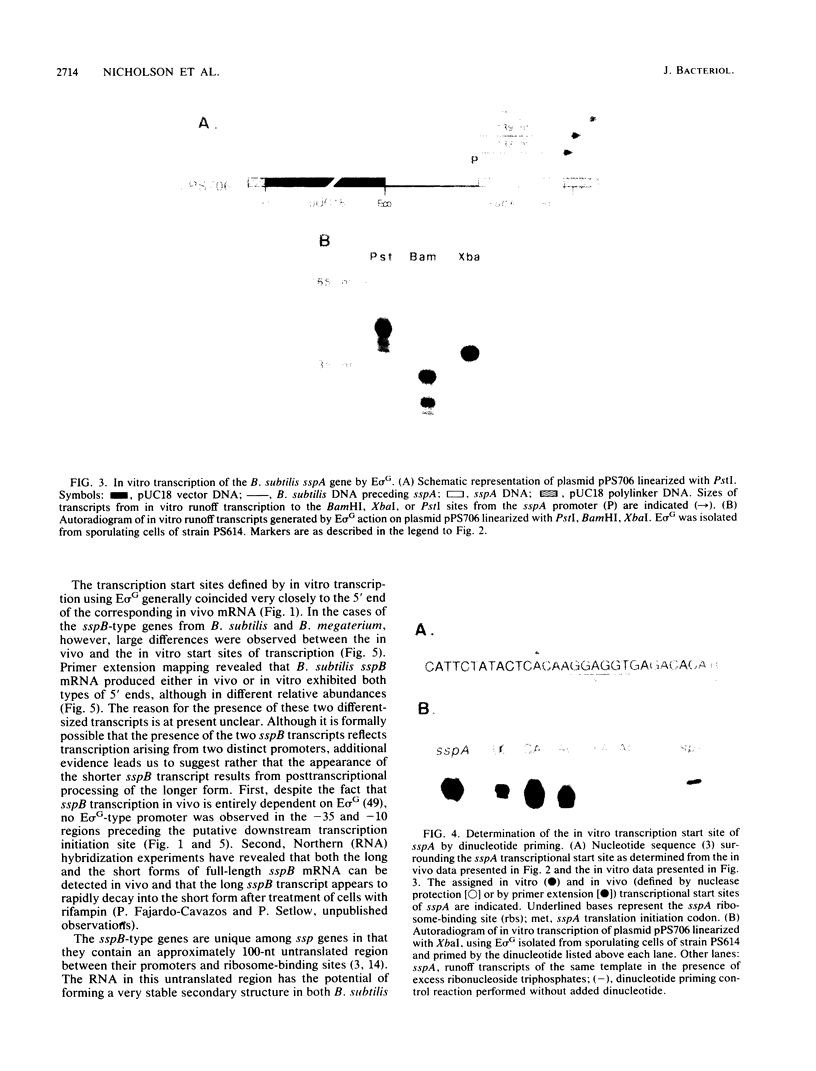
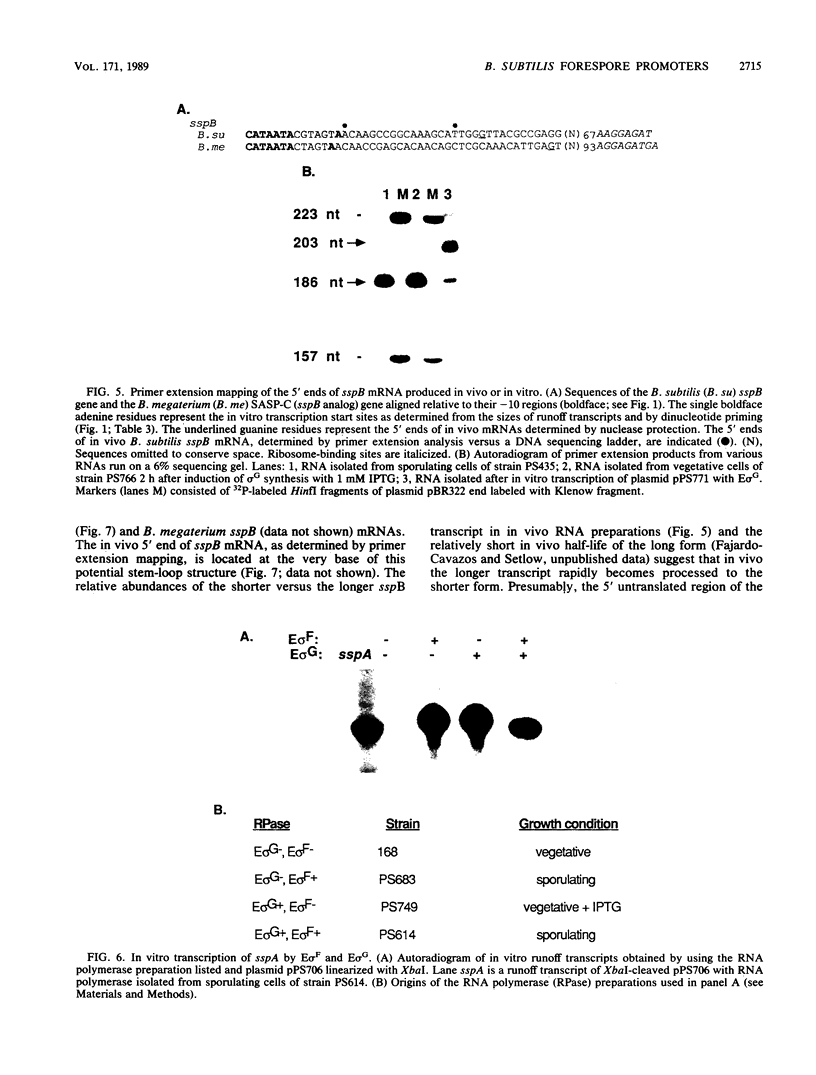
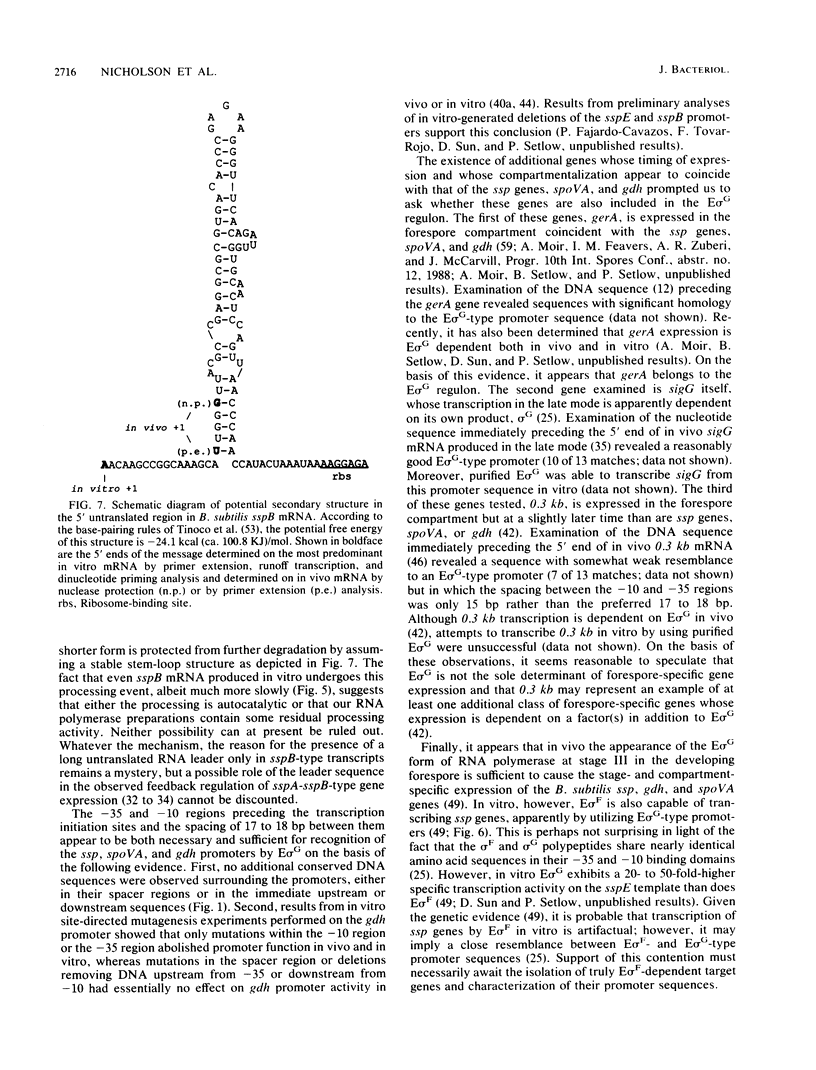
During sporulation in Bacillus subtilis, expression of the genes sspA, sspB, sspC, sspD, and sspE, which encode a family of small, acid-soluble spore proteins, as well as of the spoVA and gdh operons is transcriptionally activated at stage III of sporulation only in the forespore compartment. Transcription of these genes is mediated by RNA polymerase containing sigma G (E sigma G), the product of the sigG gene, which is itself expressed at stage III in the developing forespore. We have determined the 5' ends of transcripts generated both in vivo and in vitro by the action of E sigma G on various genes of B. subtilis and other bacilli. The 5' ends of the in vivo and in vitro mRNAs were found to coincide and were therefore considered to define the transcription initiation sites for the genes examined. We identified highly homologous DNA sequences centered at 35 and 10 base pairs preceding the transcriptional start sites of the genes examined. Consequently, we propose that these sequences define a class of promoters recognized only by E sigma G which allow transcription of genes expressed uniquely at stage III in the developing forespore.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Wang L. F., Doi R. H., Moran C. P., Jr rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M. J., Mason J. M., Setlow P. Cloning and nucleotide sequencing of genes for three small, acid-soluble proteins from Bacillus subtilis spores. J Bacteriol. 1986 May;166(2):417–425. doi: 10.1128/jb.166.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M. J., Setlow P. Cloning of a small, acid-soluble spore protein gene from Bacillus subtilis and determination of its complete nucleotide sequence. J Bacteriol. 1985 Jan;161(1):333–339. doi: 10.1128/jb.161.1.333-339.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- Dignam S. S., Setlow P. In vivo and in vitro synthesis of the spore-specific proteins A and C of bacillus megaterium. J Biol Chem. 1980 Sep 25;255(18):8417–8423. [PubMed] [Google Scholar]
- Dubnau E., Weir J., Nair G., Carter L., 3rd, Moran C., Jr, Smith I. Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol. 1988 Mar;170(3):1054–1062. doi: 10.1128/jb.170.3.1054-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2977–2985. doi: 10.1099/00221287-132-11-2977. [DOI] [PubMed] [Google Scholar]
- Feavers I. M., Miles J. S., Moir A. The nucleotide sequence of a spore germination gene (gerA) of Bacillus subtilis 168. Gene. 1985;38(1-3):95–102. doi: 10.1016/0378-1119(85)90207-0. [DOI] [PubMed] [Google Scholar]
- Fliss E. R., Loshon C. A., Setlow P. Genes for Bacillus megaterium small, acid-soluble spore proteins: cloning and nucleotide sequence of three additional genes from this multigene family. J Bacteriol. 1986 Feb;165(2):467–473. doi: 10.1128/jb.165.2.467-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Bacillus megaterium spore protein C-3: nucleotide sequence of its gene and the amino acid sequence at its spore protease cleavage site. Gene. 1984 Oct;30(1-3):167–172. doi: 10.1016/0378-1119(84)90117-3. [DOI] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Complete nucleotide sequence and start sites for transcription and translation of the Bacillus megaterium protein C gene. J Bacteriol. 1984 Jun;158(3):809–813. doi: 10.1128/jb.158.3.809-813.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Genes for Bacillus megaterium small, acid-soluble spore proteins: nucleotide sequence of two genes and their expression during sporulation. Gene. 1985;35(1-2):151–157. doi: 10.1016/0378-1119(85)90167-2. [DOI] [PubMed] [Google Scholar]
- Fort P., Errington J. Nucleotide sequence and complementation analysis of a polycistronic sporulation operon, spoVA, in Bacillus subtilis. J Gen Microbiol. 1985 May;131(5):1091–1105. doi: 10.1099/00221287-131-5-1091. [DOI] [PubMed] [Google Scholar]
- Fort P., Piggot P. J. Nucleotide sequence of sporulation locus spoIIA in Bacillus subtilis. J Gen Microbiol. 1984 Aug;130(8):2147–2153. doi: 10.1099/00221287-130-8-2147. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Ramaley R., Freese E. Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):282–293. doi: 10.1128/jb.132.1.282-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wiggs J. L., Chamberlin M. J. Nucleotide sequences of two Bacillus subtilis promoters used by Bacillus subtilis sigma-28 RNA polymerase. Nucleic Acids Res. 1981 Nov 25;9(22):5991–6000. doi: 10.1093/nar/9.22.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. H., Setlow B., Setlow P. Cloning and nucleotide sequence of the Bacillus megaterium gene coding for small, acid-soluble spore protein B. J Bacteriol. 1986 Nov;168(2):1023–1025. doi: 10.1128/jb.168.2.1023-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. H., Setlow P. Cloning, nucleotide sequencing, and genetic mapping of the gene for small, acid-soluble spore protein gamma of Bacillus subtilis. J Bacteriol. 1987 May;169(5):1985–1992. doi: 10.1128/jb.169.5.1985-1992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Karmazyn-Campelli C., Bonamy C., Savelli B., Stragier P. Tandem genes encoding sigma-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989 Feb;3(2):150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Shih C. C., Whipple F., Sonenshein A. L. Separation and analysis of the RNA polymerase binding sites of a complex Bacillus subtilis promoter. Mol Gen Genet. 1986 Aug;204(2):229–236. doi: 10.1007/BF00425503. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Loshon C. A., Fliss E. R., Setlow B., Foerster H. F., Setlow P. Cloning and nucleotide sequencing of genes for small, acid-soluble spore proteins of Bacillus cereus, Bacillus stearothermophilus, and "Thermoactinomyces thalpophilus". J Bacteriol. 1986 Jul;167(1):168–173. doi: 10.1128/jb.167.1.168-173.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Fajardo-Cavazos P., Setlow P. Levels of mRNAs which code for small, acid-soluble spore proteins and their LacZ gene fusions in sporulating cells of Bacillus subtilis. Nucleic Acids Res. 1988 Jul 25;16(14A):6567–6583. doi: 10.1093/nar/16.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986 Jul;167(1):174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda E. S., Anaguchi H., Yamada K., Kobayashi Y. Two developmental genes encoding sigma factor homologs are arranged in tandem in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7637–7641. doi: 10.1073/pnas.85.20.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Panzer S., Losick R., Sun D., Setlow P. Evidence for an additional temporal class of gene expression in the forespore compartment of sporulating Bacillus subtilis. J Bacteriol. 1989 Jan;171(1):561–564. doi: 10.1128/jb.171.1.561-564.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Moran C. P., Jr Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J Bacteriol. 1988 Nov;170(11):5086–5092. doi: 10.1128/jb.170.11.5086-5092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Stephens M. A., Lang N., Sandman K., Losick R. A promoter whose utilization is temporally regulated during sporulation in Bacillus subtilis. J Mol Biol. 1984 Jul 5;176(3):333–348. doi: 10.1016/0022-2836(84)90493-5. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bonamy C., Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988 Mar 11;52(5):697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Stragier P. Comment on 'Duplicated sporulation genes in bacteria' by J. Errington, P. Fort and J. Mandelstam. FEBS Lett. 1986 Jan 20;195(1-2):9–11. doi: 10.1016/0014-5793(86)80119-3. [DOI] [PubMed] [Google Scholar]
- Sun D. X., Setlow P. Cloning and nucleotide sequencing of genes for a second type of small, acid-soluble spore proteins of Bacillus cereus, Bacillus stearothermophilus, and "Thermoactinomyces thalpophilus". J Bacteriol. 1987 Jul;169(7):3088–3093. doi: 10.1128/jb.169.7.3088-3093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Stragier P., Setlow P. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989 Feb;3(2):141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- Sussman M. D., Vary P. S., Hartman C., Setlow P. Integration and mapping of Bacillus megaterium genes which code for small, acid-soluble spore proteins and their protease. J Bacteriol. 1988 Oct;170(10):4942–4945. doi: 10.1128/jb.170.10.4942-4945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti K. M., Moran C. P., Jr Promoter recognition by sigma-37 RNA polymerase from Bacillus subtilis. J Mol Biol. 1984 May 25;175(3):285–297. doi: 10.1016/0022-2836(84)90349-8. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Moran C. P., Jr Utilization of one promoter by two forms of RNA polymerase from Bacillus subtilis. Nature. 1985 Mar 14;314(6007):190–192. doi: 10.1038/314190a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Trempy J. E., Bonamy C., Szulmajster J., Haldenwang W. G. Bacillus subtilis sigma factor sigma 29 is the product of the sporulation-essential gene spoIIG. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4189–4192. doi: 10.1073/pnas.82.12.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zuberi A. R., Moir A., Feavers I. M. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene. 1987;51(1):1–11. doi: 10.1016/0378-1119(87)90468-9. [DOI] [PubMed] [Google Scholar]
- de Lencastre H., Piggot P. J. Identification of different sites of expression for spo loci by transformation of Bacillus subtilis. J Gen Microbiol. 1979 Oct;114(2):377–389. doi: 10.1099/00221287-114-2-377. [DOI] [PubMed] [Google Scholar]