Abstract
The nuclear receptor constitutive active/androstane receptor (CAR) is sequestered in the cytoplasm of liver cells before its activation by therapeutic drugs and xenobiotics such as phenobarbital (PB) and 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) in mouse liver, the regulatory mechanism of which remains poorly understood. Given the finding that epidermal growth factor repressed PB activation of CAR-mediated transcription (Mol Pharmacol 65:172–180, 2004), here we investigated the regulatory role of hepatocyte growth factor (HGF)-mediated signal in sequestering CAR in the cytoplasm of mouse primary hepatocytes. HGF treatment effectively repressed the induction of endogenous CYP2b10 gene by PB and TCPOBOP in mouse primary hepatocytes. On the other hand, inhibition by 1,4-diamino-2,3-dicyano-1,4-bis(methyl-thio)butadiene (U0126) of an HGF downstream kinase mitogen-activated protein kinase kinase (MEK) induced the Cyp2b10 gene and up-regulated the CAR-regulated promoter activity in the absence of TCPOBOP. HGF treatment increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 in the cytosol, thus decreasing the TCPOBOP-induced nuclear accumulation of CAR. In contrast, U0126 dephosphorylated ERK1/2 and increased nuclear CAR accumulation in the absence of TCPOBOP. These results are consistent with the conclusion that the HGF-dependent phosphorylation of ERK1/2 is the endogenous signal that sequesters CAR in the cytoplasm of mouse primary hepatocytes.
The nuclear constitutive active/androstane receptor CAR is a xeno-sensing transcription factor that regulates numerous hepatic genes in response to a large group of chemicals and therapeutic drugs. Phenobarbital (PB) represents this group of xenobiotics; it not only induces drug metabolism and secretion but also elicits pleiotropic effects on liver functions. These effects include metabolism and secretion of endobiotics (such as bilirubin), and changes in energy metabolism, cell growth, cell-cell communication, and tumor promotion (Honkakoski and Negishi, 1998a). Activation of CAR by drugs such as PB is paramount to elicit these effects by up- or down-regulating the genes that encode the key proteins and enzymes for these liver functions (Kodama and Negishi, 2006). As the function of CAR has expanded, deciphering the molecular mechanism of its activation by drugs is an urgent subject of current investigations.
Mouse hepatocytes retain CAR in the cytoplasm, thus making the nuclear translocation an initial step of its activation by drugs. CAR forms a complex with Hsp90 and co-chaperone CCRP (cytoplasmic CAR retention protein) in the cytoplasm. In response to PB, the complex recruits protein phosphatase 2A before translocation of CAR into the nucleus. The protein phosphatase inhibitor okadaic acid represses PB-induced nuclear translocation of CAR in mouse primary hepatocytes (Kawamoto et al., 1999). PB triggers the nuclear translocation without directly binding to the receptor (Swales and Negishi, 2004). These facts indicate that cellular signals are involved in regulating the cytoplasmic retention and nuclear translocation of CAR. Two observations providing insight into such a signal have been reported: the attenuation by epidermal growth factor (EGF) of CAR-mediated trans-activation of PBREM by PB and the augmentation by the MEK inhibitor U0126 of PB induction of the CYP2B gene in rat primary hepatocytes (Bauer et al., 2004; Joannard et al., 2006). MEK is a downstream protein kinase of EGF signaling and activates extracellular signal-regulated kinase (ERK). Here we have investigated the MEK-ERK signal for its ability to regulate the intracellular localization of CAR in mouse primary hepatocytes. The experimental results presented here suggest that the activation of ERK is the signal that retains CAR in the cytoplasm.
Materials and Methods
Reagents and Plasmids
PB, TCPOBOP, and ITS liquid media supplement were obtained from Sigma (St. Louis, MO); HGF was obtained from Chemicon (Temecula, CA); U0126 was obtained from Promega (Madison, WI); EGF and U0124 were obtained from Calbiochem (San Diego, CA); anti-rabbit IgG was obtained from Santa Cruz biotechnology (Santa Cruz, CA); anti-ERK1/2 and anti-phospho-ERK1/2 were obtained from Cell Signaling Technology (Danvers, MA); androstenol was obtained from Steraloids (Newport, RI). All plasmids used were previously constructed; pcDNA3 mCAR, PBREM-tk-luciferase reporter, and −1.8-kb CYP2B6 promoter in pGL3, phRL-tk (Sueyoshi et al., 1999; Zelko et al., 2001; Kobayashi et al., 2003; Swales et al., 2005).
Primary Hepatocytes
Mouse primary hepatocytes were isolated from 6- to 8-week-old Car−/− and Car+/+ male mice using a two-step collagenase perfusion as described previously (Honkakoski et al., 1996, 1998b; Yamamoto et al., 2004). Hepatocytes were suspended in Hanks’ balanced salt solution containing collagenase (0.4–0.5 mg/ml), 10 mM HEPES, pH 7.4, and 1 μM porcine insulin, collected by low-speed centrifugation, resuspended in prewarmed Williams’ E media supplemented with 7% fetal bovine serum, porcine insulin (0.1 mg/ml), transferrin (55 μg/ml), sodium selenite (50 ng/ml), streptomycin (0.1 mg/ml) and penicillin G (100 units/ml) and seeded on six-well plates or 10-cm dishes. One hour after seeding, the medium was changed to prewarmed Williams’ E media supplemented with dexamethasone (5 nM), sodium selenite (50 ng/ml), streptomycin (0.1 mg/ml), and penicillin G (100 units/ml) with or without a given chemical.
RNA Preparation and RT-PCR
Total RNAs were extracted from hepatocytes treated with PB (1 mM), TCPOBOP (250 nM), EGF (10 μg/ml), HGF (10 μg/ml), U0126 (2.5 or 25 μM), or U0124 (25 μM) for 8 h, using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNAs were synthesized using SuperScript II reverse transcriptase (Invitrogen), and real-time PCR was performed using the ABI Prism 7700 (Applied Biosystems, Foster City, CA). The CYP2B10 cDNA was amplified using 5′-AAAGTCCCGTGGCAACTTCC-3′ and 5′-TCCCAGGTGCACTGTGAACA-3′ for 5′- and 3′-primers, respectively. Amplified cDNA was measured using 6-carboxyfluorescein-ACCCCGTCCCCTGCCCCTCTT–5-carboxytetramethylrhodamine as a CYP2B10 probe. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was amplified as an internal control using the TaqMan rodent GAPDH control reagents (Applied Biosystems). The levels of a given mRNA were normalized to the GAPDH mRNA level.
Luciferease Reporter Assays
HepG2 cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum. Mouse CAR (mCAR) expression plasmids (0.1 μg) were cotransfected with PBREM-pGL3 (0.1 μg) and phRL-tk (0.1 μg) into HepG2 cells using FuGENE6 transfection reagent (Roche, Indianapolis, IN). Mouse primary hepatocytes were cotransfected with −1.8 kb-CYP2B6 promoter-pGL3 (10 μg) and phRL-tk (5 μg) using electroporation. After being treated with DMSO or a given chemical at the concentrations indicated, the cells were lysed and subjected to luciferase assays using the dual-luciferase reporter assay system (Promega, Madison, WI).
Western Blot
The nuclear extracts and cytosolic fractions were prepared from these hepatocytes using methods established previously (Dignam et al., 1983). Nuclear and cytosolic proteins were resolved on a SDS-10% polyacrylamide gel and transferred to polyvinylidene difluoride membrane using Hoefer SemiPhior (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) with Nu-PAGE transfer buffer (Invitrogen). See Blue Plus2 PreStained Standard (Invitrogen) was used as molecular weight markers. Western blot analysis was performed by using anti-rabbit IgG, anti-ERK1/2, anti-phospho-ERK1/2, or anti-CAR polyclonal antibody (Kobayashi et al., 2003). Protein bands were visualized using ECL Plus Western blotting detection reagent (GE Healthcare).
Results and Discussion
ERK-Mediated Regulation of Cyp2b10 Gene
First, we treated mouse primary hepatocytes with EGF or HGF (hepatocyte growth factor) and confirmed growth factor repression of the Cyp2b10 gene. Both EGF and HGF repressed the induction of Cyp2b10 gene by PB and TCPOBOP in the experimental conditions of our mouse primary hepatocytes (Fig. 1A). We then investigated whether or not the downstream kinase ERK1/2 played a role in this repression. Both HGF and EGF transduce their signal by activating mitogen-activated protein kinase kinase (MEK1/2) that phosphorylates ERK1/2 to further transfer the signal (Cobb et al., 1991; Seger and Krebs, 1995). U0126 is the specific MEK1/2 inhibitor commonly used to inactivate ERK1/2, thus repressing growth factor signaling (DeSilva et al., 1998; Favata et al., 1998). Mouse primary hepatocytes were treated with 2.5 or 25 μM U0126, from which cytosols were prepared for Western blot to measure the degree of the MEK inhibition as dephosphorylation of ERK1/2 using anti-phosphorylated ERK antibody. To significantly decrease the phosphorylation of ERK1/2, 25 μM U0126 was required (Fig. 1B, inset). RNAs were isolated from these hepatocytes and subjected to real-time PCR to measure CYP2B10 mRNA. In the absence of TCPOBOP, U0126 increased the mRNA 4-fold in the hepatocytes prepared from Car+/+ but not from Car−/− mice (Fig. 1B). This mRNA increase occurred only at 25 μM U0126, correlating with the dephosphorylation of ERK1/2. Taken together, growth factors seemed to repress the CAR-mediated activation of Cyp2b10 gene through the MEK-ERK pathway. As expected, TCPOBOP induced the CYP2B10 mRNA 8-fold in Car+/+ primary hepatocytes only; U0126 did not further increase this induction, suggesting that ERK regulated a step of the TCPOBOP-activation of CAR (Fig. 1B).
Fig. 1.
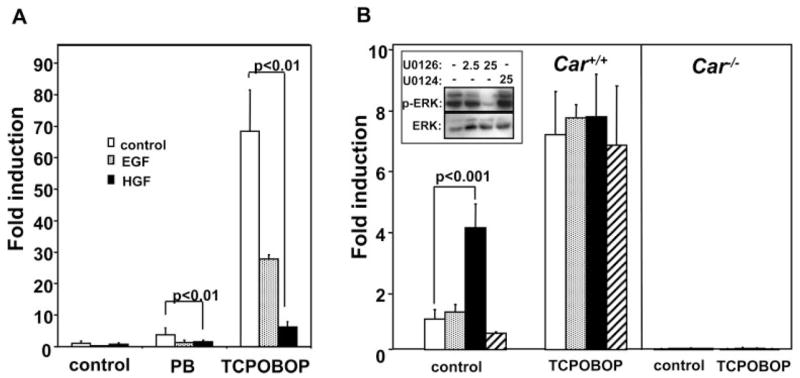
Growth factor regulation of Cyp2b gene in mouse primary hepatocytes. A, primary hepatocytes were prepared from Car+/+ mice and were treated with DMSO (open bar), EGF (10 μg/ml; stippled bar), or HGF (10 μg/ml; filled bar) in the presence or absence of PB (1 mM) and TCPOBOP (250 nM) for 8 h. From these hepatocytes, total RNAs were isolated for RT-PCR for quantitative measurement of CYP2B10 mRNA. -Fold induction was expressed by taking the corresponding value of the DMSO-treated control hepatocytes as one. Bars mean ± S.D. B, primary hepatocytes were prepared from Car−/− and Car+/+ mice and were treated with DMSO (open bar), U0126 (2.5 μM, stippled bar; 25 μM, filled bar) or U0124 (25 μM; hatched bar) in the presence and absence of TCPOBOP for 8 h. Total RNAs were prepared and subjected to RT-PCR for quantitative measurement of CYP2B10 mRNA. -Fold induction was expressed by taking the corresponding value of the DMSO-treated control hepatocytes as one. Bars mean ± S.D. The inset is Western blot showing the dephosphorylation of ERK1/2 by the MEK inhibitor U0126 and its inactive derivative U0124. The cytosols were prepared from the mouse primary hepatocytes and subjected to Western blot analyses using anti-ERK and anti-P-ERK antibodies. Numbers above bands are micromolar concentrations of inhibitors.
ERK Regulation of CAR-Mediated Transcription
The −1.8-kb CYP2B6 promoter was transfected into mouse primary hepatocytes to investigate the effect of U0126 on the CAR-mediated transcription activity. In the Car+/+ primary hepatocytes, U0126, but not U0124, activated the promoter approximately 7-fold, whereas this activation was statistically insignificant in the Car−/− primary hepatocytes (Fig. 2A). These activation patterns of CAR-mediated transcription by U0126 were reminiscent of the corresponding induction of the Cyp2b10 gene in the mouse primary hepatocytes (Fig. 1B). Because primary hepatocytes are not a suitable system to examine whether U0126 directly activates CAR, we employed transient transfection assays using the CAR-responsive enhancer module (PBREM)-Luc reporter in HepG2 cells (Fig. 2B). U0126 did not further activate the PBREM in the presence or absence of TCPOBOP. We also employed androstenol, the repressor of the constitutive activity of CAR that is routinely used to demonstrate whether a given chemical is a direct CAR activator (e.g., TCPOBOP directly binds to CAR and re-activates the androstenol-repressed CAR) (Sueyoshi et al., 1999; Tzameli et al., 2000). No re-activation of the androstenol-repressed PBREM activity was observed in the HepG2 cells (Fig. 2B). Thus, these results suggest that U0126 indirectly activated the CAR-mediated transcription.
Fig. 2.
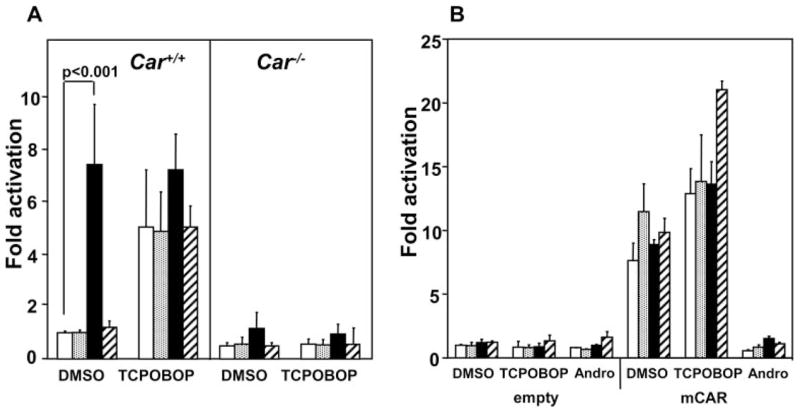
Regulation of CYP2B promoter activity by MEK-ERK in mouse primary hepatocytes. A, the −1.8-kb CYP2B6 promoter-pGL3 (10 μg) was cotransfected with phRL-tk (5 μg) into the mouse primary hepatocytes prepared from Car−/− or Car+/+ mice using electroporation and was treated with DMSO (open bar), U0126 (2.5 μM, stippled bar; 25 μM, filled bar) or U0124 (25 μM, hatched bar) in the presence or absence of TCPOBOP (250 nM) for 24 h. These hepatocytes were lysed and subjected to luciferase assays. -Fold activation was expressed by taking that of the DMSO-treated control hepatocytes as one. Bars, mean ± S.D. B, mCAR-pcDNA3 (0.1 μg) were cotransfected with PBREM-tk-Luc (0.1 μg) and phRL-tk (0.1 μg) into HepG2 cells for 24 h. Subsequently, the cells were treated with DMSO (open bar), U0126 (2.5 μM, stippled bar; 25 μM, closed bar), and/or U0124 (25 μM, hatched bar) in the presence or absence of TCPOBOP (250 nM) or androstenol (5 μM) for an additional 24 h and were lysed for luciferase assays. -Fold activation was expressed by taking the corresponding value of the DMSO-treated control hepatocytes as one. Bars, mean ± S.D.
ERK-Mediated Repression of CAR Translocation
CAR is retained in the cytoplasm of unexposed mouse primary hepatocytes and translocates into the nucleus in response to CAR activators such as PB and TCPOBOP, thus making the nuclear translocation the initial step of CAR activation (Kawamoto et al., 1999). Because CAR is spontaneously accumulated in the nucleus of HepG2 cells (Kawamoto et al., 1999) in which U0126 did not alter the CAR-mediated transcription, we examined the possibility that U0126 might inhibit the nuclear translocation of CAR in the mouse primary hepatocytes. To this end, mouse primary hepatocytes were treated with U0126 and/or TCPOBOP, from which the cytosols and nuclear extracts were prepared. Western blot analyses of the nuclear extracts showed the accumulation of CAR after the treatment with U0126 in the manner associated with the dephosphorylation of ERK1/2, whereas the nuclear CAR accumulation by TCPOBOP was independent of the function of ERK1/2 (Fig. 3). Treatment with the inactive inhibitor U0124 caused neither accumulation of CAR in the nucleus nor dephosphorylation of the ERK1/2. Thus, the ERK dephosphorylation by U0126 was sufficient to translocate CAR into the nucleus, activating the CAR-mediated transcription and inducing the Cyp2b10 gene in the absence of CAR activators. Consistent with the repressive role of ERK, HGF increased phosphorylation of ERK, thereby repressing CAR nuclear accumulation of in the mouse primary hepatocytes (Fig. 3B).
Fig. 3.
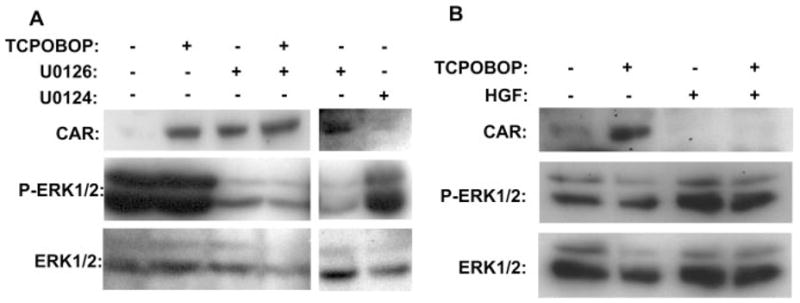
ERK-mediated regulation of CAR nuclear translocation. A, mouse primary hepatocytes were prepared from the Car+/+ mice and were treated with DMSO, TCPOBOP (250 nM), U0126 (25 μM), U0124 (25 μM), or TCPOBOP plus U0126 for 30 min. B, mouse primary hepatocytes were treated with DMSO, TCPOBOP (250 nM), HGF (10 μg/ml), or TCPOBOP plus HGF for 30 min. Both cytosols and nuclear extracts were prepared from these hepatocytes and subjected to Western blot analysis using anti-mCAR, anti-ERK1/2, and anti-phospho-ERK1/2 antibodies.
We have now shown that ERK is the signal molecule that represses the nuclear translocation of CAR in mouse primary hepatocytes. This finding explains why EGF repressed the CAR-mediated activation of CYP2B genes in rat primary hepatocytes (Bauer et al., 2004). It also agrees with the observation that U0126 augmented the induction of the CYP2B gene by PB in rat primary hepatocytes (Joannard et al., 2006). Taken in sum, this evidence is consistent with the conclusion that ERK is a repressive signal for CAR activation. Once ERK1/2 was dephosphorylated, CAR moved into the nucleus and activated the transcription of the Cyp2b10 gene in the absence of TCPOBOP (Fig. 4). If CAR retains a high constitutive activity in the primary hepatocytes, as it does in the cell-based transfection assays, then CAR should be able to activate transcription once it is in the nucleus. However, our previous work showed that the Ca+/calmodulin kinase inhibitor KN-62 did not prevent the PB-induced nuclear accumulation of CAR but could repress the induction of the Cyp2b10 gene in mouse primary hepatocytes (Yamamoto et al., 2003). Taking the opposite effects of U0126 and KN-62 into consideration, CAR requires a distinct activation signal in the nucleus, and ERK regulates only the nuclear translocation of CAR. The bulk of ERK1/2 was phosphorylated under the conditions used for culturing mouse primary hepatocytes, which might be the reason for why the dephosphorylation of ERK by PB was not detected (C. Koike, R. Moore, and M. Negishi, unpublished data). Thus, this kinase provides an excellent tool with which to further investigate the ligand-independent, signal-regulated mechanism of CAR activation by drugs, including PB.
Fig. 4.
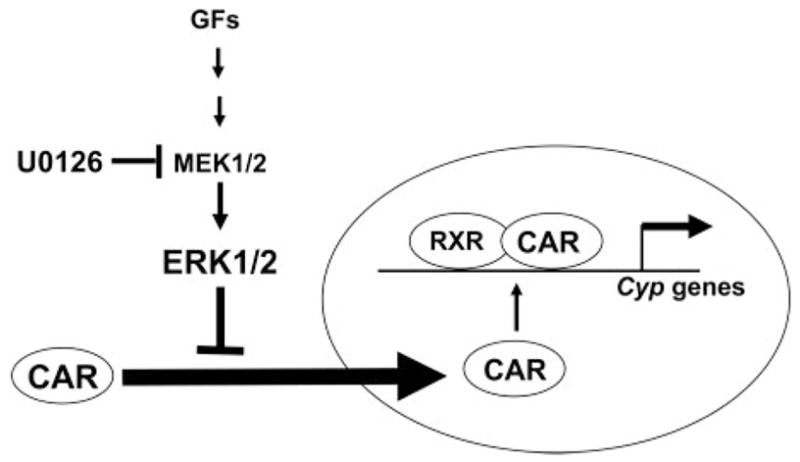
Model of MEK-ERK regulation of CAR translocation. Growth factors, such as HGF, stimulate MEK1/2 phosphorylating ERK1/2 to activate ERK1/2. Activated ERK1/2 represses the translocation of CAR into the nucleus. U0126 inhibits the activation of ERK1/2 by MEK1/2, making CAR translocate into the nucleus. In the nucleus, CAR forms a complex with the retinoid X receptor (RXR) and activates the transcription of Cyp2b10 gene in mouse primary hepatocytes.
In addition to MEK-ERK, AMP-activated protein kinase (AMPK) has recently been suggested to activate CAR to induce the CYP2B genes by PB in the human and mouse primary hepatocytes (Rencurel et al., 2005, 2006; Shindo et al., 2007). However, the process by which AMPK activates CAR is not clear at the present time. One study, using AMPK KO (α1/α2 LS−/−) mice, demonstrated that although AMPK does not regulate the PB-induced nuclear translocation of CAR, it may be involved in the activation of CAR in the nucleus (Rencurel et al., 2006). Another study demonstrated that the activation of AMPK resulted in the nuclear accumulation of CAR but was not sufficient to activate CAR-mediated transcription (Shindo et al., 2007). PB did not induce the Cyp2b10 gene in the AMPK KO mice, providing the basis of support for the notion that AMPK mediates PB induction. However, PB could not induce the Cyp2b10 gene apparently because the expression levels of the Cyp2b10 gene in the livers of nontreated AMPK KO mice were already elevated to levels near those observed in the PB-treated wild-type mice (Rencurel et al., 2006). Although AMPK is the activating signal for PB induction, MEK-ERK seems to be the repressive signal in our study. Nevertheless, whether AMPK and ERK cross-talk to generate a cellular signal for CAR activation is an intriguing question. We used the inhibitor LY294002 and found that phosphatidylinositol 3-kinase does not regulate the nuclear translocation of CAR in mouse primary hepatocytes (data not shown). PKA has also been proposed as a possible signal molecule repressing PB induction in rat primary hepatocytes (Sidhu and Omiecinski, 1995), but its molecular mechanism is poorly understood. Recent reports have suggested that the activation of protein kinases A and C modulated the pregnane X receptor-mediated induction of Cyp3a gene in mouse primary hepatocytes by altering the receptor interactions with coregulators (Ding and Staudinger, 2004a,b). Exciting research remains for the future in the signal-regulated mechanism of activation of the xenobiotic receptors CAR and pregnane X receptor.
CAR forms a complex with Hsp90 and cochaperone CCRP in the cytoplasm (Kobayashi et al., 2003). In response to PB, the complex recruits protein phosphatase 2A before translocating CAR into the nucleus, making PB-induced CAR nuclear translocation sensitive to okadaic acid, a protein phosphatase inhibitor in mouse primary hepatocytes (Kawamoto et al., 1999; Yoshinari et al., 2003). Dephosphorylation of serine 202 of mouse CAR was found to be a prerequisite for the nuclear translocation occurred (Hosseinpour et al., 2005). If ERK directly targets CAR, serine 202 can be a possible candidate of ERK phosphorylation, although the target of ERK can be the other components of the cytoplasmic CAR complex, such as CCRP and Hsp90. Identifying the phosphorylation site(s) will help us to decipher the molecular basis for the signal-regulated mechanism of CAR activation and is now the major objective of our research.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. C.K. is a Japan Society for the Promotion of Science Research Fellow in the Biomedical and Behavioral Research Program at National Institutes of Health.
ABBREVIATIONS
- CAR
constitutive active/androstane receptor
- PB
phenobarbital
- Hsp90
90-kDa heat shock protein
- CCRP
cytoplasmic CAR retention protein
- EGF
epidermal growth factor
- PBREM
phenobarbital-responsive enhancer module
- MEK
mitogen-activated protein kinase kinase
- U0124
1,4-diamino-2,3-dicyano-1,4-bis(aminophenylthio)butadiene
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
- ERK
extracellular signal-regulated kinase
- TCPOBOP
1, 4-bis[2-(3,5-dichloropyridyloxy)]benzene
- kb
kilobase pair(s)
- RT-PCR
reverse transcription-polymerase chain reaction
- HGF
hepatocytes growth factor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- mCAR
mouse CAR
- DMSO
dimethyl sulfoxide
- KN-62
1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine
- AMPK
AMP kinase
- KO
knockout
- LY294002
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
Footnotes
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
References
- Bauer D, Wolfran N, Kahl GF, Hirsch-Ernst KI. Transcriptional regulation of CYP2b1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol. 2004;65:172–180. doi: 10.1124/mol.65.1.172. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Boulton TG, Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scerle PA. Inhibition of Mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase A signal transduction pathway. J Phamacol Exp Ther. 2004a;312:849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic Cyp3A gene expression by protein kinase C. Biochem Pharmacol. 2004b;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of Mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Moore R, Gynther J, Negishi M. Characterization of phenobarbital-inducible mouse Cyp2b10 gene transcription in primary hepatocytes. J Biol Chem. 1996;271:9746–9753. doi: 10.1074/jbc.271.16.9746. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Negishi M. Regulatory DNA elements of phenobarbital-responsive cytochrome P450 CYP2B genes. J Biochem Mol Toxicol. 1998a;12:3–9. doi: 10.1002/(sici)1099-0461(1998)12:1<3::aid-jbt2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activate the phenobarbital- responsive enhancer module of the cyp2b10 gene. Mol Cell Biol. 1998b;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour F, Moore R, Negishi M, Sueyoshi T. Serine 202 regulates the nuclear translocation of constitutive active/androstane receptor. Mol Pharmacol. 2005;69:1095–1102. doi: 10.1124/mol.105.019505. [DOI] [PubMed] [Google Scholar]
- Joannard F, Rissel M, Gilot D, Anderson A, Orfila-Lefeuvre L, Guillouzo A, Atifi A, Lagadic-Gossmann D. Role for mitogen-activated protein kinases in phenobarbital-induced expression of cytochrome P450 2B in primary cultures of rat hepatocytes. Toxicol Lett. 2006;161:61–72. doi: 10.1016/j.toxlet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol. 2003;64:1069–1075. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- Kodama S, Negishi M. Phenobarbital confers its divers effects by activating the orphan nuclear receptor CAR. Drug Metab Rev. 2006;38:75–87. doi: 10.1080/03602530600569851. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, Xavier GS, Rutter GA, Viollet B, Meyer UA. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates Phenobarbital induction of cyp2b gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J. 2007;401:735–741. doi: 10.1042/BJ20061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu JS, Omiecinski CJ. cAMP-associated inhibition of Phenobarbital-induced cytochrome P450 gene expression in primary rat hepatocyte cultures. J Biol Chem. 1995;270:12762–12773. doi: 10.1074/jbc.270.21.12762. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human cyp2b6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Swales K, Kakizaki S, Yamamoto Y, Inoue K, Kobayashi K, Negishi M. Novel CAR-mediated mechanism for synergistic activation of two distinct elements within the human cytochrome P450 2b6 gene in HepG2 cells. J Biol Chem. 2005;280:3458–3466. doi: 10.1074/jbc.M411318200. [DOI] [PubMed] [Google Scholar]
- Swales K, Negishi M. CAR, driving into the future. Mol Endocrinol. 2004;18:1589–1598. doi: 10.1210/me.2003-0397. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kawamoto T, Negishi M. The role of the nuclear receptor CAR as a coordinate regulator of hepatic gene expression in defense against chemical toxicity. Arch Biochem and Biophys. 2003;409:207–211. doi: 10.1016/s0003-9861(02)00456-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi T. Identification of the nuclear receptor CAR: HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/s0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]
- Zelko I, Sueyoshi T, Kawamoto T, Moore R, Negishi M. The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xeno-chemicals in mouse liver. Mol Cell Biol. 2001;21:2838–2846. doi: 10.1128/MCB.21.8.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


