Abstract
Homers are scaffolding proteins that bind Ca2+ signaling proteins in cellular microdomains. The Homers participate in targeting and localization of Ca2+ signaling proteins in signaling complexes. However, recent work showed that the Homers are not passive scaffolding proteins, but rather they regulate the activity of several proteins within the Ca2+ signaling complex in an isoform specific manner. Homer2 increases the GAP activity of RGS proteins and PLCβ that accelerate the GTPase activity of Gα subunits. Homer1 gates the activity of TRPC channels, controls the rates of their translocation and retrieval from the plasma membrane and mediates the conformational coupling between TRPC channels and IP3Rs. Homer1 stimulates the activity of the cardiac and neuronal L-type Ca2+ channels Cav1.2 and Cav1.3. Homer1 also mediates the communication between the cardiac and smooth muscle ryanodine receptor RyR2 and Cav1.2 to regulate E–C coupling. In many cases the Homers function as a buffer to reduce the intensity of Ca2+ signaling and create a negative bias that can be reversed by the immediate early gene form of Homer 1. Hence, the Homers should be viewed as the buffers of Ca2+ signaling that ensure a high spatial and temporal fidelity of the Ca2+ signaling and activation of downstream effects.
Introduction
Homer proteins are scaffolds that play a central role in Ca2+ signaling. The Homers were discovered with the cloning of Homer1a (H1a), which is regulated as an immediate early gene. H1a is rapidly upregulated in brain neurons in response to synaptic activity induced by seizure, or during induction of long-term potentiation and is selectively induced in cells of the hippocampus when rodents engage in exploratory behavior [1, 2]. Subsequent molecular cloning and sequence searches revealed that the same Homer1 gene encodes for two additional and longer transcripts, Homer1b (H1b) and Homer1c (H1c), and revealed the presence of two additional Homer genes, Homer2 and Homer3, each of which have been reported to encode for several transcripts [3, 4].
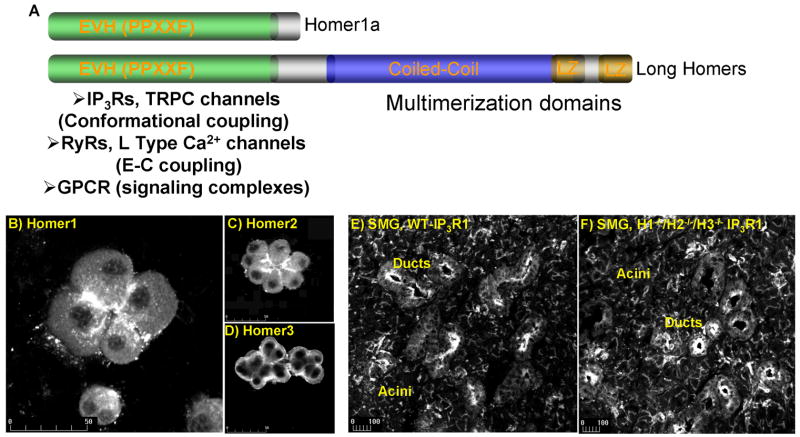
H1a consists of an EVH1 domain with a short C-terminus extension. Homer1b and 1c include the N-terminal EVH1 domain and a ~200 aa C-terminus that folds into a coiled-coil domain and two leucine zippers ([5, 6] and Fig. 1A). Homer 2 and 3 are identical in domain structure to H1b. The N terminus EVH1 domain of the different Homers displays 60–70% sequence conservation, whereas the C terminus coiled-coil domains have only about 20% sequence identity [7]. A recent structural analysis reveals that the long Homers form an elongated tetramer via their coiled-coil domains [7]. The tetrameric Homer can form a lattice with other scaffolds to bind Ca2+ signaling proteins in cellular microdomains [3, 5, 8-10]. At the same time, the monomeric H1a disrupts signaling complexes and functions as a negative regulator of the long Homers [6, 11].
Fig. 1.
Panel (A) shows the Homer domains and known interacting Ca2+ signaling proteins. Isoform-specific localization of Homer1 (B), Homer2 (C) and Homer3 (D) is demonstrated in pancreatic acini. Similar localization of type 1 IP3R in observed in WT cells (E) and cells from which all Homer isoforms were deleted. Panels (B–D) are reproduced from [30] with permission.
As scaffolding proteins, the Homers are expected to mediate assembly of complexes in cellular microdomains. Indeed, the EVH1 domain of Homers interacts with and regulates the activity of several proteins that reside in Ca2+ signaling complexes. In this short review we will discuss the role of the Homers in Ca2+ signaling with special emphasis on their active role in regulating Ca2+ signaling.
Homers localization and binding to Ca2+ signaling proteins
The role of the Homers in Ca2+ signaling became evident with the findings of the localization of the Homers in the Post-Synaptic-Density (PSD) and their interaction with the G-protein coupled metabotropic glutamate receptors (mGluRs) mGluR1 and mGluR5 [1, 2, 8]. Mutation and structural analysis revealed that the EVH1 domain binds the sequence PPXXF [12-14]. Subsequent work indicated that the EVH1 domain can also bind the sequence øPPXF and the novel ligand LPSSP [15]. In addition to the mGluRs, many Ca2+ signaling proteins express Homer ligands and bind Homer. Among them are the scaffolding protein Shank [8], PLCβ [16, 17], IP3 receptors (IP3Rs) [15, 18], TRPC channels [15, 18], ryanodine receptors (RyRs) [19-22] and selective L-type Ca2+ channel isoforms [23-25].
In addition to binding Ca2+ signaling proteins, a role for the Homers in Ca2+ signaling requires localization of the Homers within signaling microdomains. As indicated above, all Homer isoforms co-localize with mGluRs in the PSD and Homer expression is enriched in dendrites [26-29]. The localization of the Homer isoforms was further examined in the polarized pancreatic acinar cells and was found to be isoform-specific [30]. This is illustrated in Fig. 1B–1D, which shows that Homer1 and Homer2 are restricted to the apical pole, whereas Homer3 is restricted to the basal pole. Importantly, Ca2+ signaling proteins are also enriched at the apical pole and show complete co-localization with Homer1 and Homer2, but not with Homer3 [30]. These findings implicate Homer1 and Homer2, but not Homer3, in regulation of Ca2+ signaling in these cells.
The role of the Homers in assembly and localization of the Ca2+ signaling complexes is not well understood. The Homer EVH1 domain binds the Ca2+ signaling proteins, while the C-termini of mGluRs and the long H1b/c mediate targeting of the receptors to dendrites [9, 29, 31]. and the monomeric H1a disrupts this targeting [9, 31]. These targeting effects of H1b/c and H1a on mGluRs can be recapitulated by expressing the proteins in HEK cells [11] and neurons [9, 31]. In this expression system, H1b/c, but not H1a, increases the localization of mGluRs in the plasma membrane [11]. The C terminal portion of the coiled-coil domain (the CC2 region) mediates the subcellular localization of Homer itself and plays a role in clustering of mGluRs [7].
A somewhat different picture emerges from examining the role of Homers in targeting and localization of Ca2+ signaling proteins in the polarized secretory acinar cells. These studies rely on deletion of one or more Homer genes in mice and suggest that the Homers are not essential for targeting, localization or retention of Ca2+ signaling complexes in cellular microdomains [25, 30]. Several lines of evidence support this conclusion. First, deletion of the Homers enhances, rather than disrupts, Ca2+ signaling [30]. Second, deletion of the individual Homer isoforms does not affect localization of Ca2+ signaling proteins in the apical pole of polarized secretory cells [25, 30]. Finally, Figs. 1E and 1F show that even deletion of all Homer isoforms in mice does not affect the localization of IP3R1 at the apical pole of the polarized submandibular acinar and duct cells. Similarly, deletion of all Homer genes has no effect on localization of IP3R2, IP3R3, the G proteins-coupled CCK and M3 receptors or the integrity of the tight junction proteins ZO1 and ocludin-1 (unpublished results by the authors). Although these findings indicate that the Homers do not determine targeting and localization of Ca2+ signaling proteins in cellular microdomains, they do not exclude Homer-mediated control of the proximity and cross talk between the proteins. In fact, these seem to be the major roles of the Homers.
The three Homers have distinct roles in Ca2+ signaling. The best understood is the role of Homer1. It is also clear that Homer2 modulates the intensity of Ca2+ signaling. The role of Homer3 in Ca2+ signaling is not known at present. Its localization at the basal pole of secretory cells (Fig. 1D) suggests that it may not control the activity of Ca2+ signaling complexes. Indeed, preliminary studies showed that deletion of Homer3 does not affect the overall GPCR-mediated Ca2+ signaling in pancreatic acinar cells [30]. Therefore, the function of Homer3 will not be considered further in this review. We will first discuss the available information of the role of Homer2 and then the role of Homer1.
Homer 2 and RGS proteins GAP activity
The functional role of Homer2 has been examined in the brain [32, 33] and the pancreas [30]. Deletion of Homer2 in mice reveals that Homer2 is involved in appetitive pathways that underlie responses to cocaine and alcohol [32-34]. The alcohol and cocaine phenotypes of the Homer2−/− mice are consistent with enhanced response to the drugs. To understand the molecular mechanism for the enhanced responses, we analyzed Ca2+ signaling in Homer2−/− pancreatic acinar cells [30].
Deletion of Homer2 enhanced the sensitivity of GPCRs to agonist-evoked Ca2+ signals, including Ca2+ oscillations and Ca2+ waves [30]. The effect of Homer2 on the function of the biochemical (IP3 production) and biophysical (Ca2+ channels and pumps) components of the Ca2+ signal showed that Homer2 does not affect the activity of the IP3Rs, the plasma membrane Ca2+ influx channels or the activity of the ER SERCA and the plasma membrane PMCA pumps. On the other hand, Homer2 was found to regulate IP3 production [30]. GPCRs stimulate IP3 production by activating the Gq class of G proteins, which, in turn, activate of PLCβ [30].
Regulation of PLCβ by Gq-coupled GPCRs is depicted in Fig. 2A. The heteromeric Gq is composed of Gαq and Gβγ. In the resting state, Gαq is bound with GDP and is associated with Gβγ. Binding of agonist (Ag) to its receptor catalyzes the exchange of GDP for GTP to dissociate the complex and generate the active Gαq•GTP [35, 36]. Termination of the signal requires hydrolysis of GTP by the intrinsic GTPase activity of Gαq. The intrinsic GTPase activity is slow and is markedly accelerated by the RGS proteins [37, 38]. The RGS proteins are Gα GTPase activating proteins (GAP) composed of a highly conserved RGS box of about 110 amino acids and divergent C- and N- termini. The GAP activity of the RGS proteins is mediated by the RGS box [39], whereas the N-terminus domain mediates membrane targeting and confers receptor recognition [40]. Receptor recognition is mediated by binding of the N-terminus of RGS proteins and the third intracellular loop of GPCRs to the scaffolding protein spinophilin [41]. The divergent roles of the C-terminus are dependent on the motifs present in this domain [37, 38].
Fig. 2.
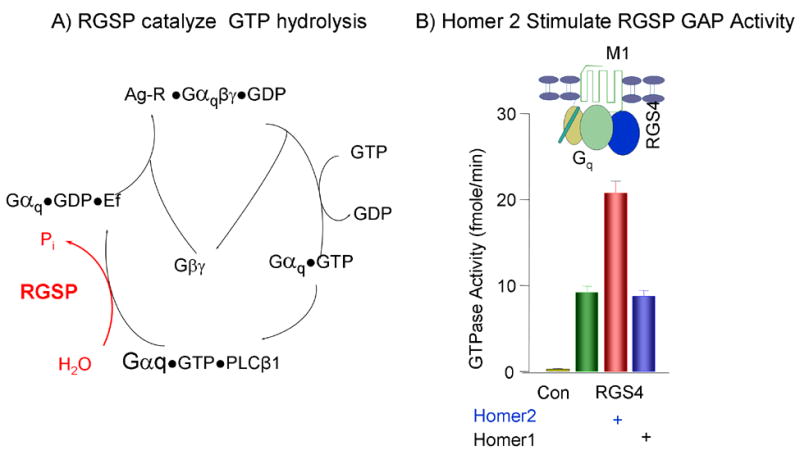
Panel (A) depicts the turn over cycle of Gq is response to receptor stimulation and the function of RGS proteins (RGSP) as Gαq GTPase activating proteins (GAP). Panel (B) show the stimulation of RGS4 GAP activity by Homer2, but not by Homer1, in reconstituted microsomes composed of Gq, the M1 receptor and RGS4 and stimulated with carbachol. The results in (B) were taken from [30] with permission.
Analysis of IP3 production in Homer2−/− cells showed that Homer2 accelerates the GAP activity of RGS proteins. Thus, deletion of Homer2 reduced the inhibitory action of RGS proteins, resulting in enhanced sensitivity to stimulation of GPCRs [30]. Fig. 2B shows that acceleration of the RGS protein GAP activity can be demonstrated in reconstituted system composed of recombinant, purified M1 muscarinic receptor, Gαq, Gβγ and RGS4. Furthermore, acceleration of the RGS protein GAP activity is specific for Homer2 since Homer1 does not have the same effect. Similarly, Homer2, but not Homer1, accelerated the GAP activity of the effector PLCβ [30].
Together, the available information indicates that Homer2 functions to tune down the intensity of Ca2+ signaling by reducing signaling by GPCRs. This is achieved by Homer2-mediated stimulation of RGS proteins and PLCβ GAP activity. Hence, Homer2 functions as a negative regulator of Ca2+ signaling by GPCRs.
Homer1 in neuronal Ca2+ signaling
Mice with deleted Homer1 have multiple neuronal abnormalities. This is not surprising by virtue of the role of Homer1 in mGluRs signaling. The effect of Homer1 in neuronal mGluRs Ca2+ signaling appears to be neuron-type specific. Homer1 binds to the proline-rich motifs PPXXF in the C terminus of mGluRs [12] and the N-terminus of the IP3Rs [15], linking them to the Shank family proteins in the PSD to form a Ca2+ signaling complex [9, 29]. Homer1 and Shank target the mGluRs to the plasma membrane when co-expressed in HeLa and other cell types [6, 8, 9, 11, 16, 17, 26]. Furthermore, when over-expressed in hippocampal neurons, the multimerizing H1b and Shank1B translocate and sequester the IP3Rs, the SERCA2b pump, calreticulin, calbindin, and portions of the ER membrane to the spines [9]. Homer proteins also affect the communication between mGluRs and the N-type Ca2+ channels in superior cervical ganglion sympathetic neurons [42]. However, this effect is likely to be indirect since the N-type Ca2+ channels do not posses Homer ligand. The Homers likely recruit the neuronal L-type Ca2+ channel Cav1.3 to the PSD since disruption of Homer complexes in striatal spiny neurons with an inhibitory peptide disrupted the modulation of Cav1.3 by stimulation of the D2 dopaminergic and M1 muscarinic receptors [24], and Cav1.3 expresses Homer binding ligand in its C-terminus.
The role of Homers in neuronal activity is less clear. For example, over-expression of H1a in Purkinje Cells decreased the rate and amplitude of the [Ca2+]i rise in response to stimulation of mGluRs [43, 44]. Similarly, H1a strongly attenuated the Ca2+ increase activated by DHPG, glutamate and NMDA in spinal cord neurons [45]. By contrast, H1a markedly increased the spontaneous activity of mGluRs and the amplitude of the mGluRs-evoked [Ca2+]i rese in cerebellar granular cells, while the long H1b strongly inhibited the response [44, 46]. These effects could be partially recapitulated by expressing the mGluRs and Homers in HEK cells, in which H1a enhanced and H1c attenuated the mGluRs-evoked Ca2+ response [47].
The reason for the opposite effects of the Homers in different neurons is not entirely clear. This can result from different degree of interruption of the mGluRs signaling complexes in the different neurons and from different composition of the signaling complexes. For example, in complexes that include Cav1.3, disruption of the complexes will affect the voltage regulated Ca2+ influx. This is not the case for mGluRs complexes that include other Ca2+ channels that do not bind Homer, such as the N-type channels. It should be possible to resolve this issue with the availability of mice from which single and combination of the Homers are deleted. The knockout mice were instrumental in discovering the acceleration of RGS proteins GAP activity by Homer2 [30] and the role of Homer1 in conformational coupling and excitation-contraction coupling (see below and [15, 18]). These studies showed that H1a facilitates, while H1b/c impedes Ca2+ signaling by regulating communication between Ca2+ release channels at the ER/SR and Ca2+ influx channels at the plasma membrane.
Homer1 and gating of TRPC channels
A role of Homer1 in the regulation of Ca2+ influx in non-excitable cells became evident when Ca2+ signaling was analyzed in Homer1−/− pancreatic acini. Deletion of Homer1 resulted in increased spontaneous Ca2+ influx [15]. Since these cells express TRPC channels and TRPC channels express the Homer binding motif PPXF in their C-terminus, we examined regulation of TRPC channels by Homer1. We also tested whether Homer1 mediates interaction between TRPC channels and IP3Rs to regulate channel gating by conformational coupling and by translocation of the channels to the plasma membrane. Indeed, Homer binds all TRPC channels and assembles a complex of TRPC channels-Homer-IP3Rs [15]. Disruption of the TRPC1 C- terminal Homer binding ligand weakens (P645L) or prevents (F648R) its binding to Homer. Analysis of the P645L mutant revealed that TRPC1 (but not other TRPC channels) expresses a second, novel, Homer binding ligand, LPSSP, in the N terminus [15]. An important finding is that disruption of the TRPC1-Homer-IP3Rs complex, by mutation in the Homer ligand or by expression of H1a, prevents the gating of TRPC1 by IP3Rs, resulting in spontaneously active TRPC1 [15]. These findings are interpreted in the model in Fig. 3. Under resting conditions TRPC channels are present in a complex with IP3Rs that is stabilized by the long H1b/c. Upon cell stimulation that upregulates H1a the complexes are dissociated to prevent inhibition by IP3Rs and increase Ca2+ influx. During the interval of H1a expression, we hypothesize that the duration of dynamic TRPC channel opening may be relatively prolonged, and Ca2+ that enters the cell maintain high Ca2+ levels just underneath the plasma membrane microdomain and may be differentially available for entry into stores.
Fig. 3.
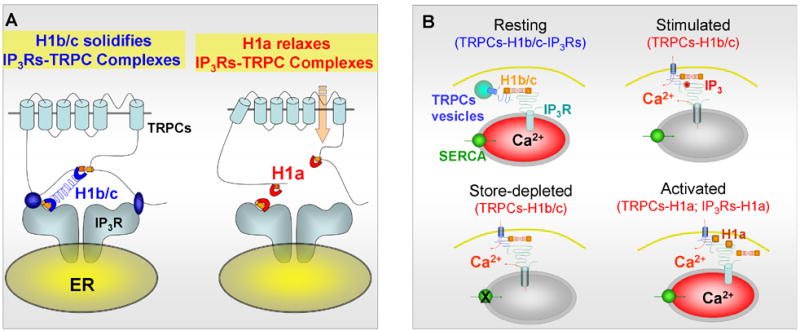
Panel (A) shows a model of a potential mechanism by which Homer1 gates TRPC channels. TRPC channels and IP3Rs have Homer binding ligands. In resting state, TRPC channels are bound to the long H1b/c through their single (all TRPCs, except TRPC1) or two (TRPC1) Homer binging ligands and to the IP3Rs. In this complex, H1b/c solidifies interaction between the channels and the channels are not active. In stress, H1a is up-regulated and replaces H1b/c. This results in relaxation of the interaction between the channels to prevent inhibition of TRPC channels activity by the IP3Rs, resulting in spontaneously active TRPC channels. Panel (B) shows a model of a potential mechanism for regulation of TRPC channels translocation by Homer1. The intracellular, vesicular pool of TRPC channels exists in TRPCs-H1b/c-IP3Rs complexes. Cell stimulation generates high level of IP3. Binding of IP3 to the IP3Rs dissociate the binding of IP3Rs to H1b/c, but H1b/c remains bound to the TRPCs. This results in activation of TRPC channels and allows the rapid re-binding of IP3Rs to H1b/c and reassembly of the complex upon termination of cell stimulation. Store depletion and H1a can also dissociate the TRPCs-H1b/c-IP3 receptors complexes to activate TRPC channels and Ca2+ influx.
Homer1 and translocation of TRPC channels
In recent years, it became clear that the activity of several TRPC channels is regulated by translocation from an intracellular pool into the plasma membrane. For the most part, receptor-mediated translocation of TRPC channels has been studied with over-expressed TRPC channels. Receptor-stimulated translocation of TRPC1 was reported to be dependent on the small GTP-binding protein RhoA, [48], which is a regulator of vesicle trafficking and exocytosis. Similarly, stimulation of the M3 receptor results in translocation of TRPC3 [18, 49] and requires the function of the SNARE protein VAMP2 [49]. Another study reported translocation of TRPC3 in response to stimulation of the EGF receptor but failed to observe translocation of TRPC3 in response to M3 receptor stimulation [50]. Stimulation of the M3 receptor causes the translocation of TRPC6 to the plasma membrane [51], and stimulation EGF receptor caused the translocation of TRPC4 [52] and TRPC5 [53] to the plasma membrane. The PI3-kinase pathway and its downstream effector Rac1 are implicated in the EGF-stimulated translocation of TRPC5 [53], which may be a general mechanism mediating Tyrosine kinase receptor mediated translocation of TRPC channels.
We asked whether the activity of the native and not only the over-expressed, TRPC channels is regulated by translocation, and the role of Homer1 in this translocation. This study showed that in resting cells, the expressed and native TRPC3 exist as TRPC3-H1b/c-IP3Rs complexes that are located, in part, at the plasma membrane and in intracellular vesicles. Receptor stimulation and binding of IP3 to the IP3Rs triggers the dissociation of the complexes. Binding of IP3 to the IP3Rs dissociates the interaction between IP3Rs and Homer1 but not between Homer1 and TRPC3 to form IP3Rs-TRPC3-H1b/c complexes. Dissociation of the complexes results in robust translocation of the TRPC3 to the plasma membrane and their retrieval upon termination of cell stimulation [18]. Analysis of TRPC3 translocation in WT and Homer1−/− pancreatic acinar cells showed that Homer1 regulates the rate of translocation and retrieval of TRPC3 channels from the plasma membrane. Hence, Homer1 has dual roles in TRPC channels function. Homer1 mediates both, the gating of TRPC channels by IP3Rs and their translocation to the plasma membrane. Fig. 3B illustrates a possible mechanism by which the assembly of the TRPC3-H1b/c-IP3Rs complexes by H1b/c mediates both the translocation of TRPC3-containing vesicles to the plasma membrane and gating of TRPC3 by IP3Rs [18].
It is interesting to note that the Homer1-regulated translocation of TRPC channels to the plasma membrane required Ca2+ depletion of intracellular stores [18]. Similarly, store-depletion is required for coalescence of stromal interacting molecule 1 (STIM1) to plasma membrane punctae and activation of TRPC channels by STIM1 [54]. It will be of interest to determine how the two TRPC channels regulatory mechanisms are related to each other and are integrated to regulate TRPC channels activity.
Homer1 and RyRs
A role of Homer proteins in E–C coupling is suggested by the presence of a Homer-binding motif in ryanodine receptors (RyRs) and the α1C subunit of Cav1.2 and α1D subunit of Cav1.3 L type Ca2+ channels [24, 25]. Indeed, measurement of Ca2+ release in permeabilized skeletal muscle fibers and the activity of RyRs reconstituted into lipid bilayers show that Homer1 activates the skeletal and cardiac muscle RyR isoforms RyR1 [19-21] and RyR2 [22]. In addition, infusion and expression of H1a, but not H1b, activates the L-type Ca2+ current in neocortex pyramidal neurons [23]. However, whether Homer1 similarly affects the channels in vivo and how these potential effects of Homer1 are translated to a role of Homer1 in E–C coupling has been resolved only recently [25].
There are three forms of E–C coupling, depending on muscle and cell type. The first type is the skeletal muscle E–C coupling, in which the skeletal muscle L-type Ca2+ channel isoform Cav1.1 is mechanically coupled to RyR1. In this form of coupling the Ca2+ release units are organized in tetrads that are formed by physical interaction between the cytosolic domains of RyR1 and Cav1.1 [55, 56]. Depolarization of the plasmalemma in the T-tubule is sensed by Cav1.1 and is mechanically conveyed to the coupled RyR1 to initiate Ca2+ release. The Ca2+ signal then propagates by a Ca2+-induced Ca2+ release (CICR) mechanism that activates RyR1 deep in the SR. In cardiac muscle, RyR2 and Cav1.2 do not physically interact. Rather, in cardiac muscle, CICR is initiated by Ca2+ influx through Cav1.2 that then activates RyR2 [57]. In the cardiac Ca2+ release units, RyR2 and Cav1.2 are in close proximity, which ensures rapid and efficient CICR [57, 58]. Several RyRs isoforms may participate in smooth muscle E–C coupling, although the predominant isoform in this muscle is also RyR2 [59, 60] and the predominant L type channel in Cav1.2 [61]. However, E–C coupling in smooth muscle displays loose coupling, as reflected by the relatively slow rate of activation of CICR [62].
The properties of E–C coupling and CICR in cardiac and smooth muscle led to the dogma that Cav1.2 and RyR2 do not directly communicate either passively or dynamically during E–C coupling. We recently examined several aspects of this notion by studying E–C coupling in the urinary bladder detrusor muscle of WT and Homer1−/− mice. We discovered a role of Homer1 in E–C coupling, in which Homer1 mediates a dynamic communication between Cav1.2 and RyR2 to reduce the intensity of CICR and leads to revision of this dogma [25]. While these studies confirmed some of the finding made in isolated system, they also revealed important differences.
A role for Homer1 in E–C coupling in vivo is evident from the aberrant urination pattern of the Homer1−/− mice. A specific effect on Homer1 in E–C coupling is suggested by the finding that the deletion of Homer1, but not Homer2 or Homer3, causes an aberrant urination pattern [25]. Measurement of muscle contraction and [Ca2+]i revealed that deletion of Homer1 increased, rather than decreased, the efficiency of E–C coupling in response to membrane depolarization and stimulation of the muscarinic receptor [25]. These findings were unexpected in view of the stimulation of Cav1.2 channel activity by Homer1 [25].
Examining the role of Homer1 in the regulation of RyR2 in vivo and of Cav1.2 in vivo and in vitro, we could not demonstrate an effect of Homer1 on the activity of RyR2. On the other hand, Homer1 directly interacts with the α1C subunit of Cav1.2 and activates Cav1.2 channel function. However, unlike the finding in neurons [23], both H1a and H1b activated the expressed Cav1.2 and the native Cav1.2 in detrusor muscle cells [25]. The activation is mediated by binding of the α1C subunit of Cav1.2 with the EVH domain of the Homers, since point mutations in the EVH1 domain that destroy binding of the PPXXF ligand to the EVH1 domain prevented activation of Cav1.2 by the Homers.
The conundrum of a lack of an apparent effect of Homer1 on RyR2 function and stimulation of Cav1.2 in vivo, yet reduced efficiency of E–C coupling in Homer1−/− detrusor muscle, was solved by examining the role of Homer1 in the communication between Cav1.2 and RyR2. Expression of RyR2, Cav1.2 in HEK cells resulted in CICR. Co-expression of H1a with the RyR2 and Cav1.2 enhances, whereas co-expression of H1b with the RyR2 and Cav1.2 reduces the efficiency of CICR, similar to the findings with WT and Homer1−/− detrusor muscle strips [25]. Tagging the Homer motif-expressing N-terminus of RyR2 and C terminus of Cav1.2 with a complementary N and C halves of GFP was used to show that H1a prevents and H1b mediates the interaction between RyR2 and Cav1.2 [25]. Importantly, the Homer1-mediated interaction between RyR2 and Cav1.2 is dynamic in that it changes in response to membrane depolarization and receptor stimulation and correlates with the role of Homer1 on CICR [25].
It is interesting to note that the urinary bladder detrusor muscle and the cardiac muscle express the same RyR2 and Cav1.2 isoforms [57, 59-61], and both muscle types express Homer1 [63]. It is thus likely that the two muscle types are regulated by the same mechanisms. This implies that Homer1 also regulates cardiac function. Future testing of cardiac function in Homer1−/− mice should reveal whether Homer1 participates in cardiac function.
The in vitro and in vivo findings define the molecular basis of a “2-state” model by which Homer1-mediated interaction between Cav1.2 and RyR2 regulates E–C coupling to reduce the responsiveness of the muscle to cell stimulation. This is illustrated in the model in Fig. 4 5. In state one, Cav1.2 couples to RyR2 by H1b/c to reduce the sensitivity of activation of CICR by membrane depolarization. In state two, H1a uncouples Cav1.2 and RyR2 to enhance responsiveness to membrane depolarization to induce CICR. This model predicts that in smooth muscle cells that upregulate H1a, such as might occur in response to vascular injury or irritation of the bladder, agonists and membrane depolarization will result in enhanced contraction. If so, this mechanism could contribute importantly to vasospasm or irritable bladder syndrome. Understanding the dynamic nature of the effect of Homer1 on the interaction between the channels should clarify important aspects of E–C coupling in cardiac and smooth muscle function.
Fig. 4.
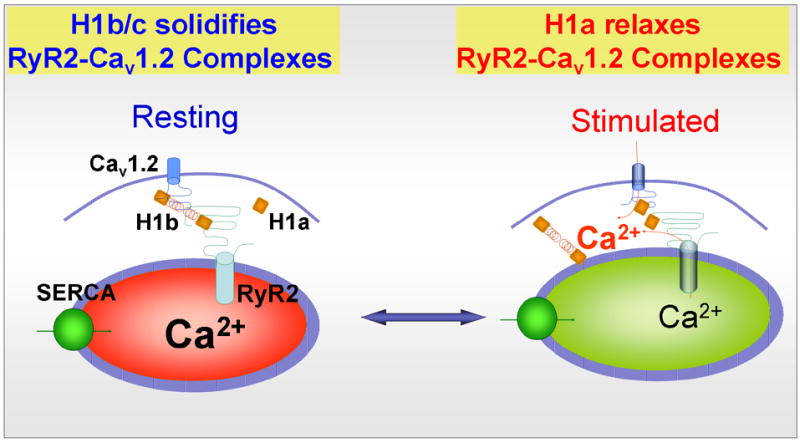
Homer1 regulates E–C coupling in a mechanism equivalent to that of conformational-coupling found for TRPC channels. H1b/c binds Cav1.2 (and Cav1.3 in neurons) and RyR2 to form the complex Cav1.2-H1b/c-RyR2. In this complex H1b/c solidifies the interaction between the channels to hinder the conformational transmission between Cav1.2 and RyR2 in response to membrane depolarization and reduce muscle (and neuronal) excitability. When H1a is up-regulated, it binds to the channels to relax the interaction between Cav1.2 and RyR2 and facilitate the conformational transmission between Cav1.2 and RyR2. Furthermore, H1a activates Cav1.2 (and Cav1.3) to further enhance Ca2+ influx and activation of CICR. The two effects of H1a result in increase muscle (and neuronal) excitability and increasing spontaneous contraction (and perhaps firing of spontaneous action potentials).
The Homers as negative regulators of Ca2+ signaling
The findings summarized in this short review indicate that the Homers are unique adaptors or scaffolding proteins that are involved not only in the targeting and localization of Ca2+ signaling proteins (such as the mGluRs), but also in regulating the activity of several Ca2+ signaling proteins that reside within the Ca2+ signaling complex (such as RGS proteins, TRPC channels, Cav1.2, Cav1.3 and RyRs). The Homers regulate the function of diverse forms of Ca2+ signaling in both excitable and non-excitable cells. In many cases the Homers function as negative regulators that reduce the intensity of Ca2+ signaling. In the case of GPCR-mediated Ca2+ signaling, the Homers act on the two components of the Ca2+ signal. Homer2 controls the biochemical component of the Ca2+ signal by increasing the activity of the inhibitors of Gαq to reduce IP3 production. Homer1 controls the biophysical component of the Ca2+ signal by solidifying the interaction between the Ca2+ release and influx channels and reduce their activation. These effects of the Homers suggest that the Homers function as buffers of Ca2+ signaling. Buffering activation of the Ca2+ signal has the important effect of reducing the spontaneous activity of Ca2+ signaling complexes and high spatial and temporal fidelity of the Ca2+ signaling and activation of downstream effects. Such a function highlights the critical role of Homer proteins in Ca2+ signaling by excitable and non-excitable cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–75. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- 2.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–8. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 3.Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–4. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 4.Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Molecular characterisation of two structurally distinct groups of human homers, generated by extensive alternative splicing. J Mol Biol. 2000;295:1185–200. doi: 10.1006/jmbi.1999.3436. [DOI] [PubMed] [Google Scholar]
- 5.Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proc Natl Acad Sci U S A. 1999;96:13801–6. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–16. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J Neurosci. 2006;26:8492–501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 9.Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–92. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 11.Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–7. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- 12.Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26:143–54. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 13.Irie K, Nakatsu T, Mitsuoka K, Miyazawa A, Sobue K, Hiroaki Y, Doi T, Fujiyoshi Y, Kato H. Crystal structure of the Homer 1 family conserved region reveals the interaction between the EVH1 domain and own proline-rich motif. J Mol Biol. 2002;318:1117–26. doi: 10.1016/S0022-2836(02)00170-5. [DOI] [PubMed] [Google Scholar]
- 14.Barzik M, Carl UD, Schubert WD, Frank R, Wehland J, Heinz DW. The N-terminal domain of Homer/Vesl is a new class II EVH1 domain. J Mol Biol. 2001;309:155–69. doi: 10.1006/jmbi.2001.4640. [DOI] [PubMed] [Google Scholar]
- 15.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–44. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JI, Kim HS, Lee JR, Kim E, Ryu SH, Suh PG. The interaction of phospholipase C-beta3 with Shank2 regulates mGluR-mediated calcium signal. J Biol Chem. 2005;280:12467–73. doi: 10.1074/jbc.M410740200. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–9. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 19.Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, Pessah IN. Homer regulates gain of ryanodine receptor type 1 channel complex. J Biol Chem. 2002;277:44722–30. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003;34:177–84. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 21.Ward CW, Feng W, Tu J, Pessah IN, Worley PK, Schneider MF. Homer protein increases activation of Ca2+ sparks in permeabilized skeletal muscle. J Biol Chem. 2004;279:5781–7. doi: 10.1074/jbc.M311422200. [DOI] [PubMed] [Google Scholar]
- 22.Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium. 2003;34:261–9. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Sakagami Y, Sugiura S, Inokuchi K, Shimohama S, Kato N. Homer 1a enhances spike-induced calcium influx via L-type calcium channels in neocortex pyramidal cells. Eur J Neurosci. 2005;22:1338–48. doi: 10.1111/j.1460-9568.2005.04278.x. [DOI] [PubMed] [Google Scholar]
- 24.Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–62. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang G, Kim JY, Dehoff M, Mizuno Y, Kamm KE, Muallem S, Worley PF, Zeng W. Ca2+ signaling in microdomain: Homer1 Mediates the Interaction between RyR2 and Cav1.2 to regulate E–C coupling. Journal of Biological Chemistry. 2007 doi: 10.1074/jbc.M611529200. in press. [DOI] [PubMed] [Google Scholar]
- 26.Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–6. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T. Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci. 2003;22:188–201. doi: 10.1016/s1044-7431(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–9. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE, 2002. 2002:RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- 30.Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–9. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- 32.Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–61. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalivas PW, Szumlinski KK, Worley P. Homer2 gene deletion in mice produces a phenotype similar to chronic cocaine treated rats. Neurotox Res. 2004;6:385–7. doi: 10.1007/BF03033313. [DOI] [PubMed] [Google Scholar]
- 34.Szumlinski KK, Toda S, Middaugh LD, Worley PF, Kalivas PW. Evidence for a relationship between Group 1 mGluR hypofunction and increased cocaine and ethanol sensitivity in Homer2 null mutant mice. Ann N Y Acad Sci. 2003;1003:468–71. doi: 10.1196/annals.1300.055. [DOI] [PubMed] [Google Scholar]
- 35.Freissmuth M, Casey PJ, Gilman AG. G proteins control diverse pathways of transmembrane signaling. Faseb J. 1989;3:2125–31. [PubMed] [Google Scholar]
- 36.Gilman AG. G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 37.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 38.Ishii M, Kurachi Y. Physiological actions of regulators of G-protein signaling (RGS) proteins. Life Sciences. 2003;74:163–71. doi: 10.1016/j.lfs.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Popov S, Yu K, Kozasa T, Wilkie TM. The regulators of G protein signaling (RGS) domains of RGS4, RGS10, and GAIP retain GTPase activating protein activity in vitro. Proc Natl Acad Sci U S A. 1997;94:7216–20. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng W, Xu X, Popov S, Mukhopadhyay S, Chidiac P, Swistok J, Danho W, Yagaloff KA, Fisher SL, Ross EM, Muallem S, Wilkie TM. The N-terminal domain of RGS4 confers receptor-selective inhibition of G protein signaling. J Biol Chem. 1998;273:34687–90. doi: 10.1074/jbc.273.52.34687. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nature Cell Biology. 2005;7:405–11. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 42.Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–45. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–26. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 44.Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur J Neurosci. 2003;17:1023–32. doi: 10.1046/j.1460-9568.2003.02499.x. [DOI] [PubMed] [Google Scholar]
- 45.Tappe A, Klugmann M, Luo C, Hirlinger D, Agarwal N, Benrath J, Ehrengruber MU, During MJ, Kuner R. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat Med. 2006;12:677–81. doi: 10.1038/nm1406. [DOI] [PubMed] [Google Scholar]
- 46.Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–5. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 47.Abe H, Misaka T, Tateyama M, Kubo Y. Effects of coexpression with Homer isoforms on the function of metabotropic glutamate receptor 1alpha. Mol Cell Neurosci. 2003;23:157–68. doi: 10.1016/s1044-7431(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 48.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 49.Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC, Combs C, Das S, Leenders AG, Sheng ZH, Knepper MA, Ambudkar SV, Ambudkar IS. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell. 2004;15:635–46. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Smyth JT, Lemonnier L, Vazquez G, Bird GS, Putney JW., Jr Dissociation of regulated trafficking of TRPC3 channels to the plasma membrane from their activation by phospholipase C. J Biol Chem. 2006;281:11712–20. doi: 10.1074/jbc.M510541200. [DOI] [PubMed] [Google Scholar]
- 51.Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–6. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- 52.Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–87. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- 53.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–20. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 54.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 55.Franzini-Armstrong C. Functional implications of RyR-dHPR relationships in skeletal and cardiac muscles. Biol Res. 2004;37:507–12. doi: 10.4067/s0716-97602004000400003. [DOI] [PubMed] [Google Scholar]
- 56.Paolini C, Protasi F, Franzini-Armstrong C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J Mol Biol. 2004;342:145–53. doi: 10.1016/j.jmb.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 57.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 58.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 59.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca(2+) sparks in smooth muscle. J Gen Physiol. 2004;123:377–86. doi: 10.1085/jgp.200308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wegener JW, Schulla V, Koller A, Klugbauer N, Feil R, Hofmann F. Control of intestinal motility by the Ca(v)1.2 L-type calcium channel in mice. Faseb J. 2006;20:1260–2. doi: 10.1096/fj.05-5292fje. [DOI] [PubMed] [Google Scholar]
- 62.Kotlikoff MI. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog Biophys Mol Biol. 2003;83:171–91. doi: 10.1016/s0079-6107(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 63.Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267:634–9. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]