Abstract
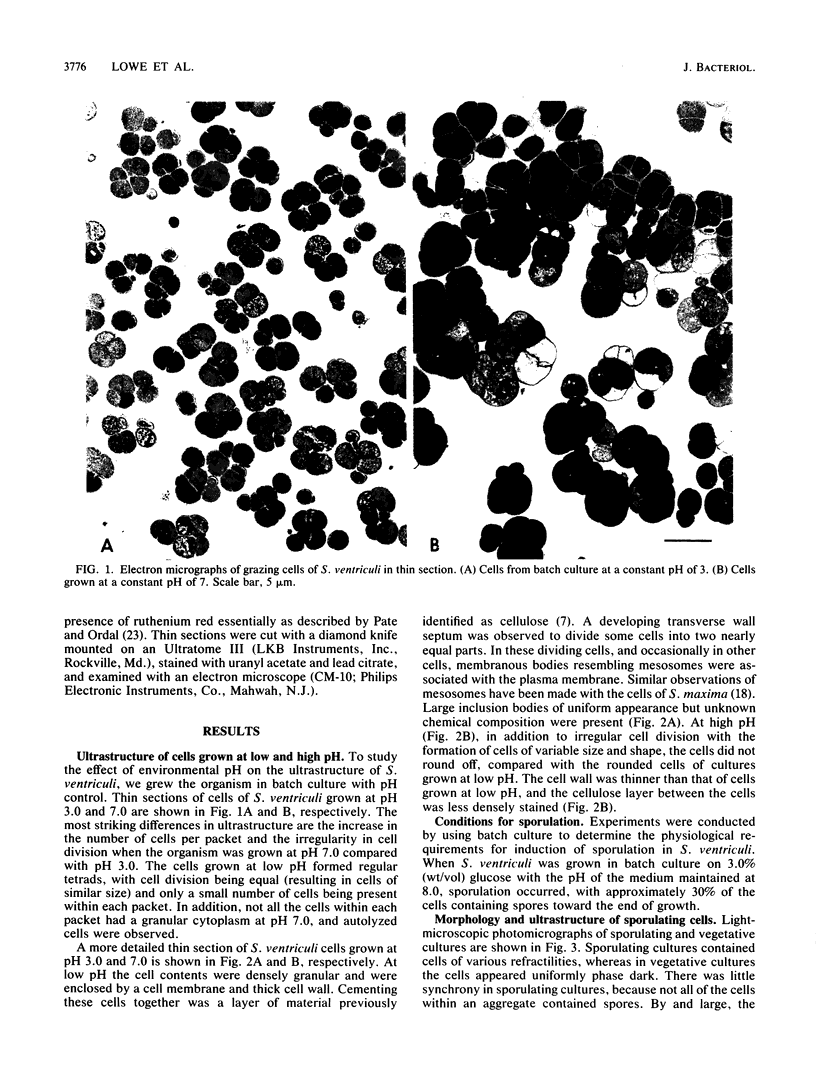
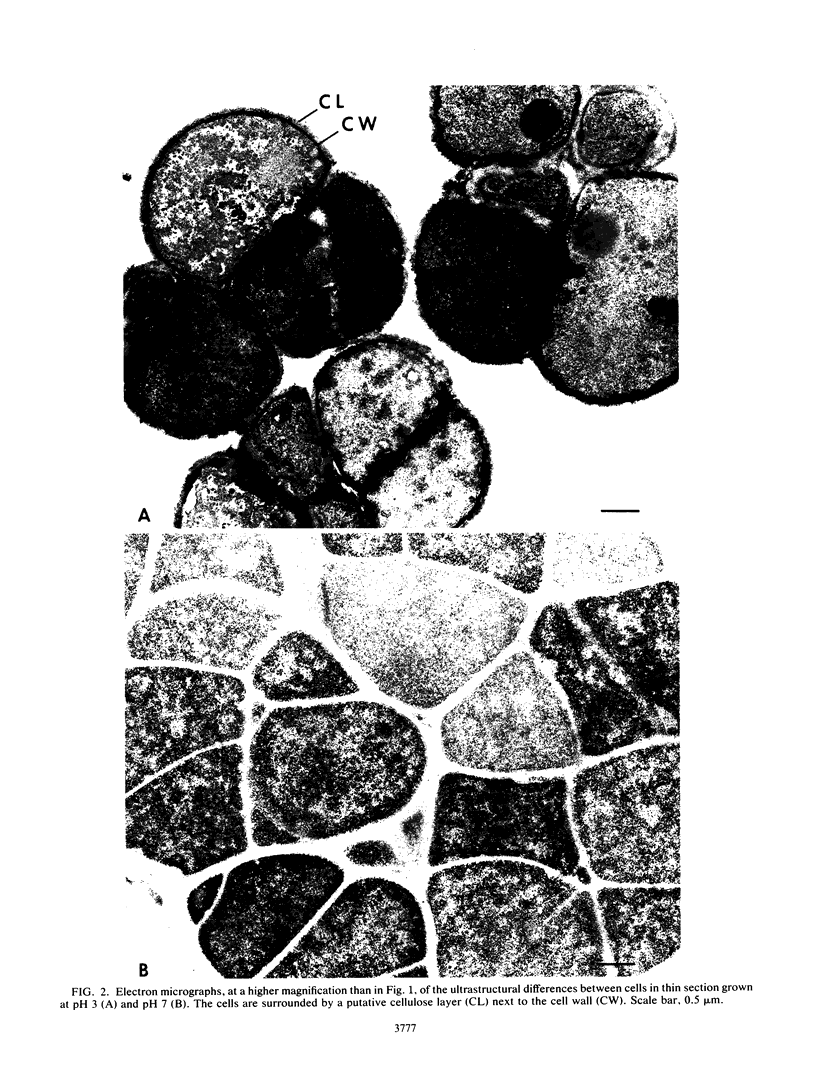
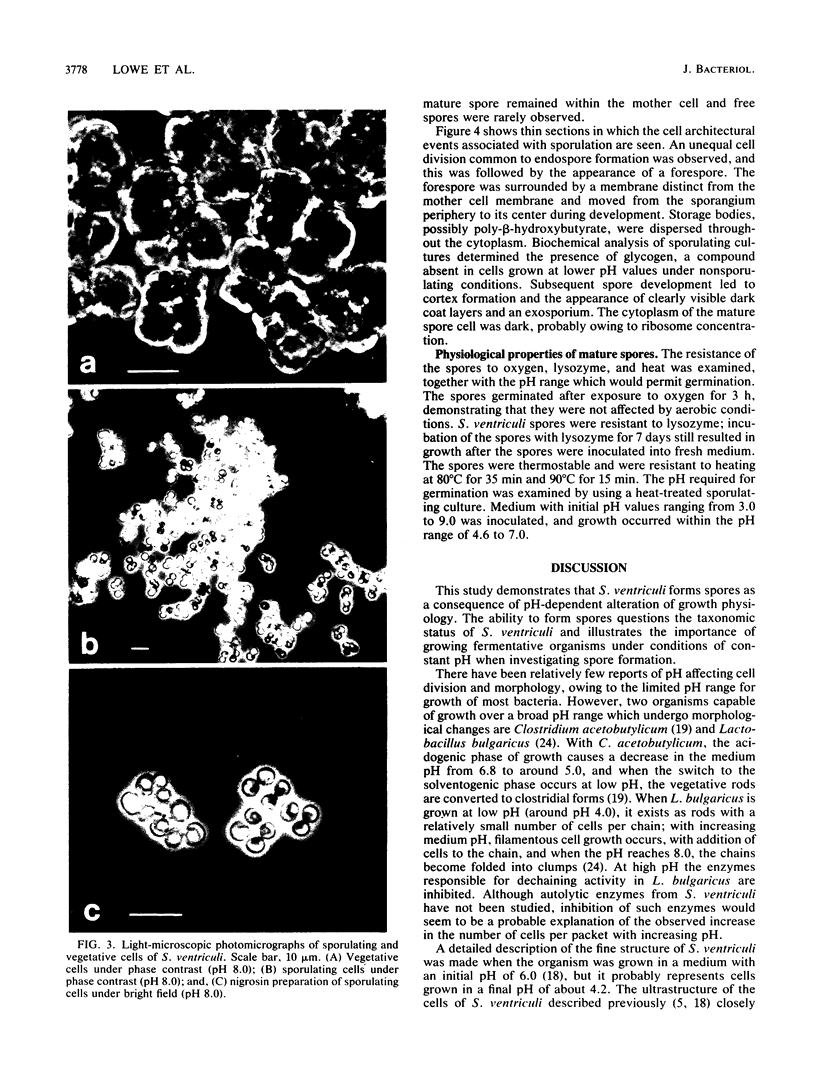
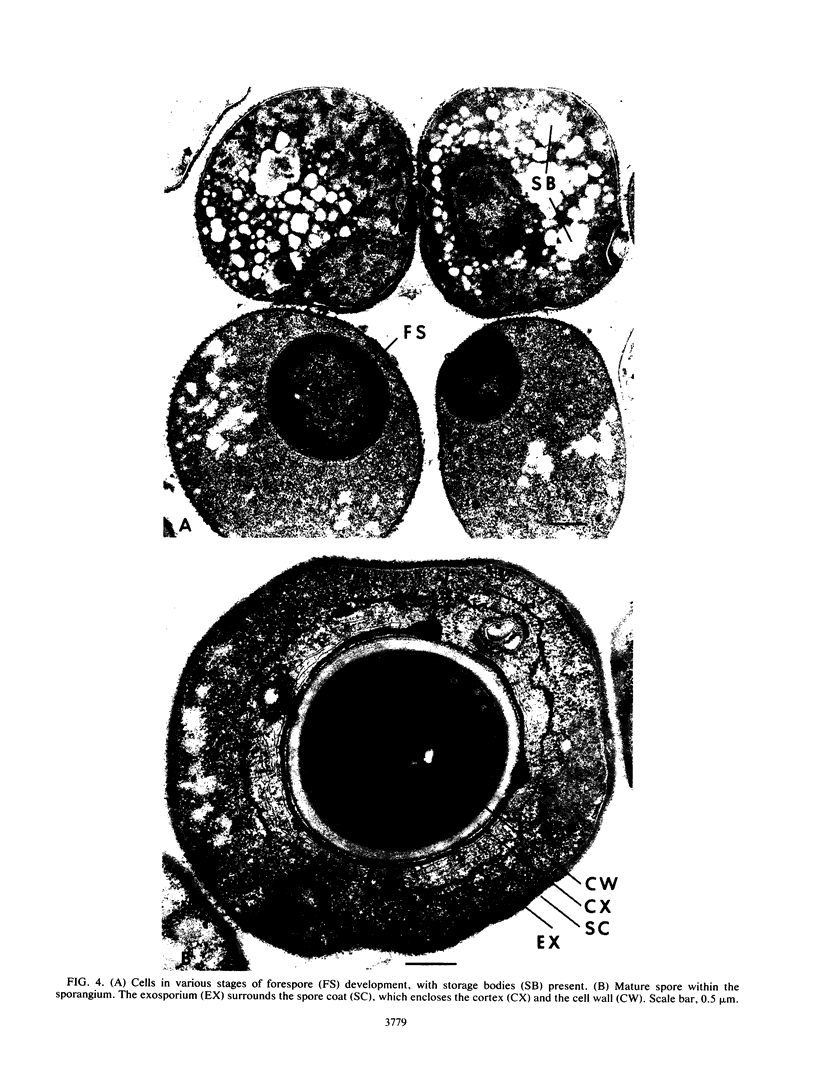
Distinct morphological changes in the ultrastructure of Sarcina ventriculi were observed when cells were grown in medium of constant composition at pH extremes of 3.0 and 8.0. Transmission electron microscopy revealed that at low pH (less than or equal to 3.0) the cells formed regular packets and cell division was uniform. When the pH was increased (to greater than or equal to 7.0), the cells became larger and cell division resulted in irregular cells that varied in shape and size. Sporulation occurred at high pH (i.e., greater than or equal to 8.0). The sporulation cycle followed the conventional sequence of development for refractile endospores, with the appearance of a cortex and multiple wall layers. The spores were resistant to oxygen, lysozyme, or heating at 90 degrees C for 15 min. Spores germinated within the pH range of 4.6 to 7.0.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALE-PAROLA E., BORASKY R., WOLFE R. S. Studies on Sarcina ventriculi. III. Localization of cellulose. J Bacteriol. 1961 Feb;81:311–318. doi: 10.1128/jb.81.2.311-318.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALE-PAROLA E., WOLFE R. S. Studies on Sarcina ventriculi. I. Stock culture method. J Bacteriol. 1960 Jun;79:857–859. doi: 10.1128/jb.79.6.857-859.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Biology of the sugar-fermenting Sarcinae. Bacteriol Rev. 1970 Mar;34(1):82–97. doi: 10.1128/br.34.1.82-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S., Zeikus J. G. Ecophysiological adaptations of anaerobic bacteria to low pH: analysis of anaerobic digestion in acidic bog sediments. Appl Environ Microbiol. 1987 Jan;53(1):57–64. doi: 10.1128/aem.53.1.57-64.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S., Zeikus J. G. Physiological adaptations of anaerobic bacteria to low pH: metabolic control of proton motive force in Sarcina ventriculi. J Bacteriol. 1987 May;169(5):2150–2157. doi: 10.1128/jb.169.5.2150-2157.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., NAYLOR H. B. Studies of the fine structure of microorganisms. II. Electron microscopic studies on sporulation of Clostridium sporogenes. J Bacteriol. 1958 Jun;75(6):647–653. doi: 10.1128/jb.75.6.647-653.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger J. F., Stuart P. F., Holt S. C. Cytology of spore formation in Clostridium perfringens. J Bacteriol. 1968 Nov;96(5):1818–1834. doi: 10.1128/jb.96.5.1818-1834.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Sarcina maxima and Sarcina ventriculi. J Bacteriol. 1967 Jan;93(1):399–410. doi: 10.1128/jb.93.1.399-410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on chain length of lactobacillus bulgaricus. J Bacteriol. 1980 Dec;144(3):865–868. doi: 10.1128/jb.144.3.865-868.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K. E., Vaughn R. H. Exosporium formation in sporulating cells of Clostridium botulinum 78A. J Bacteriol. 1972 Oct;112(1):618–621. doi: 10.1128/jb.112.1.618-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]